As we look around we can make observations about the things around us. we might note things such as color, smell, or roughness. These observations are considered physical characteristics or properties of matter. These physical properties of matter include the following: color, odor, state of matter at room temperature, density, solubility, heat and electrical conductivity, hardness and boiling and freezing points. If you look at the table on page 641 in your textbook you will find properties of some common elements. These are poperties that help us to identify substances. Each substance has a density unique to itself. Density is the relationship between mass and voume os a given substance. If two object share the same abount of space but object "a" has a greater mass than object "b", then object "a" is more dense. "More stuff packed in a certain an=mount of sapce. An objects buoyancy is determined by comparing densities. For example, Water has a density of 1g/cm3. If an object has a density less than that of water then it will float. Conversely, if an object has a density great than that of water it will sink.
Sometimes substances can be mixed together in either a homogeneous or heterogeneous mixture. These substances still retain their chemical properties. We can use magnets to separate iron from a mixture. We can also filter out large rocks from a handful of sand. Ohter processes of separating subsances include settling (letting your chocolate milk sit for a while and the chocolate goes to the bottom of the glass), and evapration (when you dry in the sun after you go swimming in the ocean salt is left behind on your skin).
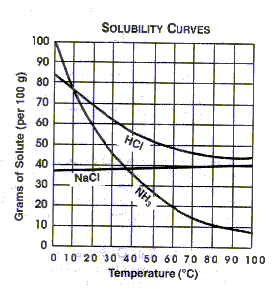
How substances mix or become soluable in water or liquids can depend on the temperature of the liquid. For example, look at the graph below. This graph shows the soluability of various substances in water. By looking at the graph we can see the as the temperature increases the amount of HCl that can be dissolved goes down.
These substances can be either a soild, liquid or gas at room temperature. Below is a chart of three states of matter and chacrteristics of each phase.