Surface tension can be created more completely by knowing the energetics of creating and cracking of molecular neighbors. This may be done by something called calorimetry, or the measure of heat flow during an process called isothermal .
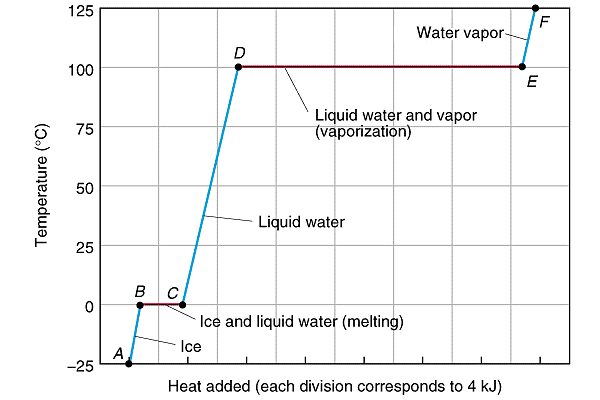
The boiling of a sample of water from -25 to 130 Celcuis involves both, the heat capacities of the phasaes but as well as the enthalpies of the melting, and boiling of water.