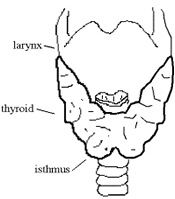
Anatomy & Development
The thyroid gland weights about 30 grams and is highly vascularized. The functional unit of the thyroid gland is the follicle: a microscopic spherical sac lined mostly by follicular cells. Each gland has about 3 million follicles that produce the thyroid hormones T3 and T4. Follicular cells are cuboidal and facilitate many of the processes that lead to formation of thyroid hormone inside the follicle. Another cell type, clear cells (aka parafollicular or C cells), dispersed thought the lining of the follicle and between follicles produce calcitonin, an hormone involved in calcium homeostasis. Also interspersed between the follicels are sympathetic nerve ending, capillaries and limphatic vessels.
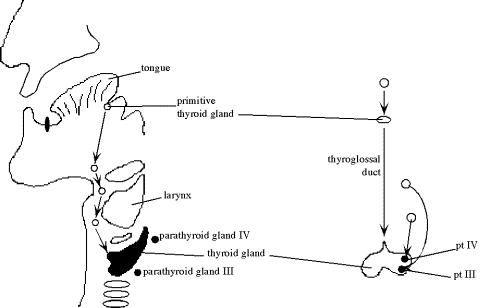 Most
of the thyroid arises from primitive gastrointestinal track (endoderm) that
migrates from the mouth area down towards the neck through a path known as the
thyroglossal duct. But clear cells come from the neural crest (ectoderm).
Most
of the thyroid arises from primitive gastrointestinal track (endoderm) that
migrates from the mouth area down towards the neck through a path known as the
thyroglossal duct. But clear cells come from the neural crest (ectoderm).
Common developmental abnormalities of the thyroid include: thyroglossal duct remains as active thyroid tissue (~ 15% population); thyroid does not descend fully; or it is compleatelly absent. The thyroid is often asymmetric, with the right lobe being larger than the left. The female thyroid is larger than the male's, and it increases in size during puberty, pregnancy and lactation, and according to the seasons.
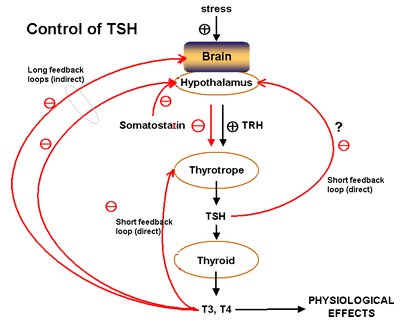
The pituitary hormone Thyroid Stimulating Hormone (TSH) promotes general growth of the thyroid gland as well as many steps in thyroid hormone synthesis.

Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Topic 1
Topic 2
Advance Topics: Topic 1
Topic 2
 Thyroid
Hormones
Thyroid
Hormones
The two main thyroid hormones are thyroxine (T4) and 3,5,3'-triiodo-L-thyronine (T3).
Follicular cells trap iodide (I![]() )
by active transport from the circulation and synthesize thyroglobulin (TGB)
a large glyoprotein (5000 aa, ~ 660,000 mol.wt.) with about 125 tyrosine residues.
TGB is composed of two weakly associated
identical subunits, about 10% carbohydrate and 1% iodine.
)
by active transport from the circulation and synthesize thyroglobulin (TGB)
a large glyoprotein (5000 aa, ~ 660,000 mol.wt.) with about 125 tyrosine residues.
TGB is composed of two weakly associated
identical subunits, about 10% carbohydrate and 1% iodine.
Iodide uptake by the sodium/iodide symporter protein (NIS) is activated by TSH. NIS is a 12-transmembrane span protein. Thyroid follicle cells sequester iodide against a 30x gradient. The mechanism was unclear until 1996 (Nature 379: 458, 1996). Old theories speculated a lipid-mediated mechanism.
TGB is made in the rough ER and packaged into secretory vessicles in the golgi for release into the colloid. Once inside follicular cells, the iodide is oxidated to iodine (I2) by peroxidase, and then diffuses through the membrane into the colloid.
2I![]() --- peroxidase ---> I2
--- peroxidase ---> I2
Two classes of compounds that prevent iodine uptake by the thyroid are monovalent ions and thiocarbamides. Monovalent ions like perchlorate and thiocyanate block accumulation of of iodide by the thyroid. Thiocarbamates like thiourea and thiouracil inhibit iodide oxidation and iodination-coupling reactions (TPO?).
In the colloid, peroxidase also catalyses the addition of iodine to the aromatic ring of tyrosines in TGB, thus yielding monoiodotyrosine (T1) and diiodotyrosine (T2). Then peroxidase also catalizes the joining of two T2 or one T2 and one T1 to yield T4 or T3, respectivelly. About one third of the tyrosine residues in TGB are available for reaction.
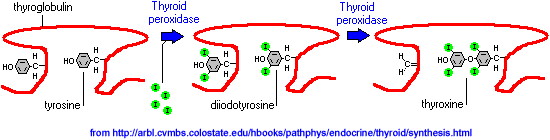
TSH stimulates T3 and T4 synthesis on thryroglobulin and colloid engulfment into droplets by follicular cells.
The colloid droplets that enter the follicular cell are digested by intracellular proteases, thus releasing T3 and thyroxine. The proteases come from fussion of lysosomes with the colloid droplets, in response to TSH activity. Thyroxine and T3 then diffuse into the bloodstream, this process also controlled by TSH. Any T1 or T2 undergo deiodination catalyzed by iodothyrosine dehalogenase, and the iodides are recycled.
Thyroxine is normally secreated in greater quantity than T3, but T3 is more potent. Thyroxine is converted to T3 in target tissues by 5-deiodinase.
T4 ---
5-deiodinase ---> T3 + I![]()
In the circulations, thyroid hormones are strongly bound to serum binding proteins (thyroid binding proteins, TBPs). Only 0.015% of T4 and 0.33% of T3 remain free in the circulation. Thyroxyne binding globin (TBG) has high affinity for the hormones. Thyroxine binding prealbumin (TBPA) has moderate affinity for the porteins. Serum albumin has a low affinity.
Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Topic 1
Topic 2
Advance Topics: Topic 1
Topic 2
Thyroid Hormone Receptors
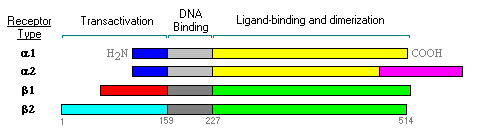 The
thyroid receptors, TRa1, c-erbAa2,
TRb1 and TRb2, are nuclear
receptor transcription factors that bind to Thyroid Response Elements (TREs)
in DNA. An example of a gene controlled by a TRE is the TSHb
gene expressed in pituitary thyrotrope cells.
The presence of multiple forms of the thyroid hormone receptor, with tissue
and stage-dependent differences in their expression, suggests an extraordinary
level of complexity in the physiologic effects of thyroid hormone.
The
thyroid receptors, TRa1, c-erbAa2,
TRb1 and TRb2, are nuclear
receptor transcription factors that bind to Thyroid Response Elements (TREs)
in DNA. An example of a gene controlled by a TRE is the TSHb
gene expressed in pituitary thyrotrope cells.
The presence of multiple forms of the thyroid hormone receptor, with tissue
and stage-dependent differences in their expression, suggests an extraordinary
level of complexity in the physiologic effects of thyroid hormone.
TRa1 and TRb1 are found in many tissues and binds T3. c-erbAa2 is found in many tissues but does not bind T3. TRb2 is found only in the pituitary and hypothalamus, and binds T3. All the thyroid receptors dimerize with the retinoid X receptors: RXRa, RXRb, and RXRg. TRs are mostly nuclear, even in the absence of ligand.
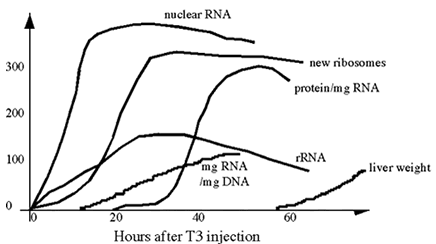 To
study the effects of thyroid hormone in vivo, a thyroidoctomized rodent can
be injected with low doses of T3. The RNA and protein synthesis is measured.
To
study the effects of thyroid hormone in vivo, a thyroidoctomized rodent can
be injected with low doses of T3. The RNA and protein synthesis is measured.
Many actions of the thyroid hormones in mammals are permissive. They regulates growth in conjuction with growth hormone by directing early neural development, and bone growth and development. They act as "homeostatic controls" stimulating feeding behaviour, increasing metabolic rate, oxygen consumption, and heart rate, and decreasing liver glycogen and plasma cholesterol. To produce thermogenesis, thyroid hormones increase mitochondrial oxygen consumption and ATP synthesis. Thyroid hormones are also required for liver conversion of carotenes to vitamin A, and in a variety of tissues induce many enzymes and other cellular processes. It is required by the pituitary for synthesis of prolactin and growth hormone. Finally, it excerts a negative feedback on TSH production from the pituitary. T3, T4 may also be direct and/or indirect signals in promotion of apoptosis.
Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Topic 1
Topic 2
Advance Topics: Topic 1
Topic 2
Thyroid Hormone in Amphibians
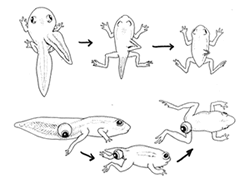
Thyroid hormone is key on amphibians metamorphosis from an aquatic juvenil to a terrestrial adult. There are two general classess of amphibians according to their developmental pattern: anurans (tailes amphibians, e.g. frogs, toads), and urodele (tailed, e.g. newsts, salamanders, axolots).
 Anurans
loose their tail and gills, and gain legs and lungs as they undergo metamorphosis.
They also develop ticker, dryer skin and change from being hervivorous to being
carnivorous, and accordingly develop a new mouth with a sticky tongue. The adults
also develop eyelides, eye reflexes, ocular muscles and a different kind of
hemoglobin, and their liver/kidney protein metabolite excreted changes from
ammonia to urea. ALl of this is done while feeding and moving: there is no quiesent
stage.
Anurans
loose their tail and gills, and gain legs and lungs as they undergo metamorphosis.
They also develop ticker, dryer skin and change from being hervivorous to being
carnivorous, and accordingly develop a new mouth with a sticky tongue. The adults
also develop eyelides, eye reflexes, ocular muscles and a different kind of
hemoglobin, and their liver/kidney protein metabolite excreted changes from
ammonia to urea. ALl of this is done while feeding and moving: there is no quiesent
stage.
Anurans change from premetamorphic tadpole (without limbs), to prometamorphic tadpole (with hind legs), to tadpole (with four limbs and a large tail), to froglet (still has a small tail) to mature frog (fully formed limbs, no tail). As they move through the stages of metamorphosis, the leves of TR and thyroid hormone expression increase, to decrease when the adult stage has been reached. TRa and RXRa reache peak expression early in development and stays high throught metamorphosis. TRb and thyroid hormone slowly increase in the early stages but reach a sharp peak in the latter stages, a period knwon as metabolic climax (?).

Urodeles can be paedomorphic, i.e. they reach sexual maturity while keeping juvenile appearance (neoteny). This depends on temperature. For example the tiger salamander (Ambystoma tigrinum) is paedomorphic and aquatic in high, cold areas, but metamophose and are land dwellers in low, warm areas. Axolotls (Ambystoma mexicanum) normally do not metamorphose but can be induced to metamorphose with thyroid hormones. Some salamanders cannot be induced.

 If
a frog is injected with frog pituitary extract, there is an increase in T4 release
from the thyroid. But if a frog is injected with axolot pituitary extract, there
is no change at lower concentrations and a decrease in T4 release at the highest
concentration. Therefore, axolots probably have a "defect" in either
TRH or TSH release.
If
a frog is injected with frog pituitary extract, there is an increase in T4 release
from the thyroid. But if a frog is injected with axolot pituitary extract, there
is no change at lower concentrations and a decrease in T4 release at the highest
concentration. Therefore, axolots probably have a "defect" in either
TRH or TSH release.
In an experiment to determine the organ specificity to thyroid hormone responses in anuran metamophosis, either a tail or an eye from a metamophosing froglet is grafted on the back of another metamorphosing froglet. The grafted tail eventually dissapears, but the grapfted eye does not change. Therefore, hormonal responses vary amoung target organs at different stages of metamorphosis, and receptors and their cofactors must be organ-specific.
Prolactin may play an opposing role to thyroid hormone in amphibian development. There is a low TH/PRL ratio during premetamorphosis, then levels of prolactin seem to decrease as TH levels increase during prometamorphosis, to finally achieve a high TH/PRL ratio at metamorphic climax.
Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Topic 1
Topic 2
Advance Topics: Topic 1
Topic 2
Diseases
Hypothyroidism is caused by a deficiency of thyroid hormones (hypothyroxinemia). It is a common disease: about 1 in 4000 people are affected. Primary hypothyroidism refers to failure of thyroid hormonal function, and may be due to familial or congenital thyroid dysgenesis (usually genetic causes). Secondary hypothyroidism is due to a pituitary or hypothalamic defect.
Childhood hypothyroidism is known as cretinism and may be either goitrous cretinism (lack of iodide in diet) or athyreotic cretinism (congenital absence of thyroid). Adult hypothyroidism is known as myxedema and may be due to simple goiter (lack of iodide in diet) or primary myxedema (disfunction of the thyroid gland). Idiopathic primary myxedema is due to thyroiditis, an inflamation of the thyroid gland, often of unknown cause. Iatrogenic primary myxedema is due to surgical removal or chemical inactivation of the thyroid gland. Spontaneous primary myxedema is due to and autoimmune disorder like Hashimoto's thyroiditis.
Familial end-organ resistance to thyroid hormones, low T3 syndrome, and thyroid gland resistance to TSH may also cause myxedema. There is a familial TBG deficiency syndrome but usually does NOT cause hypothyroidism. Mutations in the sodium/iodide symporter protein (NIS) may underlie thyroid diseases, e.g. thyroid cancer (?).
Possible clinical symptoms of hypothyroidism include weight gain, goiter (visibly enlarged thyroid gland), puffy appearance, loss of hair, low basal metabolic rate (BMR), low body temperature, decreased perspiration, lethargy, depression, and intolerance to cold. Hypothyroidism is usually treated by taking T4.
Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Topic 1
Topic 2
Advance Topics: Topic 1
Topic 2
![]()
![]() Continue
to "Gut Hormones" or take a test:
[T1] [T2] [T3].
Continue
to "Gut Hormones" or take a test:
[T1] [T2] [T3].
Need more practice? Answer the following review questions:
Questions not yet available