
Welcome
to the Homepage of
Dr. William Antonio Boyle
at Prince George's Community College,
Maryland, USA.

Chemistry, CHM 1010, Section LR02, M, W - DAYTIME - HYBRID.
Chemistry, CHM 1020, Section LE01, M - EVENING - IN-PERSON.
Chemistry, CHM 1030 (Lab) Section LE01, W - EVENING - IN-PERSON.
Spring Semester classes will start the week of January 20th, 2026.
Chemistry, CHM 1010, Section HL01 - EVENING- HYBRID.
Chemistry, CHM 1030 (Lab) Section LD01 - DAYTIME - IN-PERSON.
Fall Semester Final Exams will be on December 4th, 2025.
Dr. Boyle's
schedule - Fall Semester 2025:
| Hour | Monday | Tuesday | Wednesday | Thursday | Friday |
| 10:00 AM | |||||
| 11:00 AM | CHM1030 Lec LD01 11:00-11:50am CHES 316 | ||||
| 12:00 AM | CHM1030 Lab LD01 | ||||
| 1:00 PM | 12:00-2:50 pm CHES 316 | ||||
| 2:00 PM | |||||
| 3:00 PM | |||||
| 4:00 PM | Office Hours 4:00-5:00pm REMOTE | Office Hours 4:00-5:00pm REMOTE | Office Hours 4:00-5:00pm REMOTE | Office Hours 4:00-5:00pm REMOTE | |
| 5:00 PM | CHM1010 Lec HL01 | CHM1010 Lab HL01 | |||
| 6:00 PM | 5:00-7:30pm CHES 114 | 5:00-8:15pm CHES-312 | |||
| 7:00 PM | or REMOTE | ||||
| 8:00 PM | CHM1010 Rec HL01 8:00-8:50pm | ||||
| 9:00 PM | |||||
| 10:00 PM |
Chemistry 1010, Fall Semester 2025 - Tentative Discussion/Laboratory
Schedule --
|
Week/Dates |
Lecture/Discussion Topics |
Lab
(Thu) |
Assessments |
|
1. 08/18-08/21 |
Introduction
to Chemistry Measurement |
Safety
and Equipment |
|
|
2. 08/25-08/28 |
Properties
and Classification of Matter Elements, Compounds, Mixtures |
Measurements |
HW: 01.01, 01.02, 01.03 |
|
3.
09/01-09/04 |
Symbols,
formulas Physical
vs chemical changes Conservation
of matter Chemical Nomenclature |
Kinds
of Matter Separations I |
HW: Ch. 01* StudyPlan. |
|
4. 09/08-09/11 |
Chemical Reactions Assign Project |
Investigation of Solutions |
HW: |
|
5.
09/15-09/18 |
The
Mole Concept Stoichiometry |
Performance Lab #1 |
|
|
6.
09/22-09/25 |
Energy |
Chemical Reactions 1 |
HW:Ch. 03 StudyPlan. |
|
7. 09/29-10/02 |
Atomic Structure and Periodic Trends |
Moles. Molecules, Formulas |
Exam 1; HW: |
|
8. 10/06-10/09 |
Bonding |
Chemical Reactions 2 |
HW:Ch. 05 StudyPlan. |
|
9.
10/13-10/16 No class
on 10/14 |
Molecular
Geometry, VSEPR Hybridization |
Performance Lab #2 |
HW: |
|
10.
10/20-10/23 |
Gas Laws |
Ins and Outs of Energy in Systems |
Element Project due. HW: |
|
11. 10/27-10/30 |
Properties of Liquids and Solids |
Exploring Acids and Bases |
Exam 2, HW: |
|
12. 11/03-11/06 |
Solution
Concentrations Colligative
Properties |
Behavior of Gases |
HW: |
|
13.
11/10-11/13 |
Acids and Bases |
Spectroscopy |
Exam
3, HW: |
|
14.
11/17-11/20 |
Intermolecular Forces |
Performance Lab #3 |
HW: |
|
15. 11/24-11/27 |
Phase Changes |
(no Lab!) |
|
|
16. Final Exam |
In-person. |
Section HL01 |
Th Dec 04 6:00-8:20pm |
(*Chapter in Moore/Stanitski)
* * * * *
The Chemistry 1010 Syllabus.
The Chemistry 1010 Supplement to Syllabus.
| Login to Canvas. |
Canvas instructions. |
Chemistry 1030, Fall Semester 2025 - Tentative Lab/Discussion Schedule --
|
Week/Dates |
Topics |
Assessments |
|
1. 08/18-08/21 |
Introduction to Lab Safety/ Types of errors and significant figures |
Safety Quiz |
|
2. 08/25-08/28
|
Use of Analytical Balance
|
Sig.Fig. Quiz Lab report |
|
3. 09/01-09/04
|
Using excel and
Linear Regression in |
Quiz
|
|
4. 09/08-09/11
|
Measurement Variation |
Lab report |
|
5. 09/15-09/18
|
Accuracy & Precision |
Performance Task#1 |
|
6. 09/22-09/25 |
More Lights, Color and Absorption |
Lab report |
|
7. 09/29-10/02 |
Conductometric Measurement |
Lab Report |
|
8. 10/06-10/09 |
Characterization of Weak Acid |
Lab report |
|
9. 10/13-10/16 No class on 10/14 |
Mid-Term |
Mid-Term |
|
10. 10/20-10/23
|
Titrimetric Analysis of Antacid |
Performance Task#2 |
|
11. 10/27-10/30 |
Determination of Crystal Violet |
Lab report |
|
12. 11/03-11/06
|
Spectroscopic Determination of Equilibrium Constant for an Ion Complex |
Performance Task #3 |
|
13. 11/10-11/13
|
Investigation of Electrochemical Reactions |
Lab report |
|
14. 11/17-11/20
|
Culminating Chemical Analysis |
Performance Task#4 |
|
15. 11/24-11/27
|
No class! Thanksgiving Day! |
|
|
16. Final Exam |
Thursday, Dec. 4
|
Final Exam (Cumulative) 11:00 am – 1:20 pm |
** Performance Lab Reports are due the same day of the lab (end of the lab).
All other lab reports are due before the midnight the day of the lab.
The Chemistry 1030 Syllabus.
The Chemistry 1030 Supplement to Syllabus.
| Login to Canvas. |
Canvas instructions. |
The CHM 1010 Element Project guidelines.
A report with active hyperlinks: Example #1.
A report with active hyperlinks: Example #2.
A report with active hyperlinks: Example #3 (You will need to download the report for the hyperlinks to work).
A report with active hyperlinks: Example #4 (You will need to download the report for the hyperlinks to work).
Handouts:
Stoichiometry/Limiting Reactant Problem.
Stoichiometry/Limiting Reactant Problem Solution.
Listing of Common Ions.
The energy consumption problem.
The energy consumption problem solution.
The radiation and cancer problem.
The radiation and cancer problem solution.
An example separation procedure.
Rules for Writing Lewis Structures of Molecules.
Student log-on and payment instructions for the OWLv2 system.
Dr. Scott Sinex's Chemistry 1010 webpage.
Dr. Barbara Gage's Chemistry 1010 topics review webpage.
The Chemistry 1010 Lab Manual.
The Khan Academy Tutoring Videos.
The Library of Congress AsK A Librarian.
PGCC Student Scholarship Search and Application Site!
Free US College Student Scholarship and Grant Information!
Free Transfer College and Scholarship Search Information.
Courses taught by Dr. Boyle at Prince George's Community College:
Dr. Boyle has been an Adjunct Professor at PGCC since the summer of 2003.
Prince George's Community College Example Student Projects:
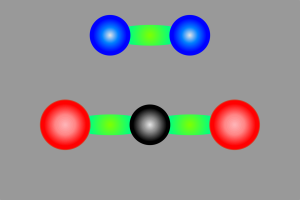
Gas molecules of 3 or more atoms, such as CO2 or CH4 (Carbon dioxide, methane, and others)
can BEND or FLEX and ABSORB heat radiation -- and act as GREENHOUSE gases, trapping the Sun's heat!
Honors:
Dr. Boyle's academic background:
![[Professor Boyle with his Fish Viewing Chamber]](images/WBFVCh.jpg)
| Professor Boyle in 1992 with the prototype Fish Viewing Chamber he designed and built. The Fish Viewing Chamber allowed sharp images of the fish to be taken by the video camera on top as the fish were pumped through the chamber (The live aquaculture fish are unharmed by this routine pumping procedure). Professor Boyle also designed the image-processing algorithm to obtain the weight from images of the flexible fish bodies as they tumbled through the tubing and chamber, i.e., in any pose and viewed from any angle; not a trivial proposition. The motivation for this research was an optimization of each individual aquaculture farm's profits: To minimize the costs of fish feed (the largest cost item for aquaculture farming) while maximizing the growth of the farmed fish (marketable product). The research project was a spectacular technical success; this computer-vision system measured the weights of the fish with an accuracy of within one percent. |
![[Geometrical analysis of fish images]](images/ProjGeo.gif)
![[Flow diagram for the production of Pekilo protein]](images/PekFlowC.jpg)
| An application of biotechnology: The Flow Diagram for the material and energy balances for the production of 680.3 kilograms per hour of Pekilo (Paecilomyces sp.) dried protein concentrate. The byproduct carbohydrate feed stream is concentrated with a nanofilter and is then sent to a continuous aerobic bioreactor where it is transformed into Pekilo protein biomass. The Pekilo protein biomass slurry exiting the bioreactor is then filtered, pressed, and dried into the dry Pekilo protein concentrate product. This product is used in the formulation of livestock feed and is also used for human protein supplements. Similar biotechnology processes are used for the manufacture of many different products, from ethanol for beverages or biofuel, to monoclonal antibodies for medical uses. |
"What is Energy?" --WAB.
"Inertia is also a property of gray matter." --WAB.
Back to the Natural
Sciences Department webpage
* * * * *
Thank you for your visit!