The way in which the components of associative learning - classical conditioning and operant conditioning - contribute to learning in the fruit fly Drosophila melanogaster, has been extensively studied by Brembs (2000), Brembs and Heisenberg (2001) and Heisenberg, Wolf and Brembs (2001), in experiments using a flight simulator.
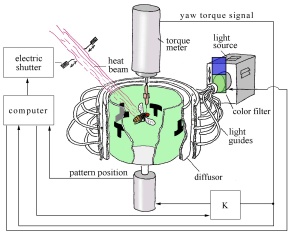
Fruit flies display many kinds of behavioural flexibility in their patterns of bodily movement, or "ethogram". In the experiments, the range of the fruit flies' bodily movements was drastically restricted in a highly unnatural fashion. Each fly, after being immobilized by cold-anaesthesia and glued by the head and thorax to a triangular copper hook, was left overnight with some food (sucrose) in a small moist chamber. The next day, it was attached to a torque meter via a clamp and performed tethered flight in the centre of a cylindrical panorama. The torque meter measured the fly's tendency to turn around its vertical body axis - i.e. its yaw torque. The fly was held horizontally, as if cruising at high speed. The head was glued to the thorax. The fly was unable to turn or shift its gaze or its orientation, but was free to bend its abdomen, extend its proboscis, beat its wings and move its legs. The torque meter to which the fly was attached measured the difference between the fly's left and right wing movements, and interpreted this as an "intended" side-to-side turn.
Most of the experiments were designed to monitor the flexibility of a single behavioural variable in the fly: yawing (movement from side to side). Fruit flies, when allowed to fly freely, do not fly at just one angle, but spontaneously vary their angle of flying over the entire yaw torque range. Fluctuations in yaw torque may be slow or rapid (jerky body movements, known as "spikes"). Torque spikes, generated when the fly reduces its wing beat amplitude on the side to which it "intends" to turn, enable the fly to make sudden turns to the left or right. In the experimental set-up, when the fly "attempted" to make a side-turn, it did so by generating torque spikes.
The flight simulator used in the experiments was a computer-controlled feedback system in which a fly was allowed to control, by its yaw torque, the rotations of a cylindrical panorama surrounding it. In some experiments, the cylindrical arena was used to present a visual stimulus - either a colour or a pattern (a conditioned stimulus). The fly's tendency to perform left or right turns (yaw torque) during tethered flight was fed into the computer. The computer controlled the background visual display of colors and/or patterns perceived by the fly. Thus although the fly could not actually turn left or right, the rotations of the panorama could be used to provide the fly with artificial sensory feedback from its "intended" turns, which mimicked the feedback it would receive in free flight from turning. It also regulated a heat beam which could be focused onto the fly. The heat beam served as an unconditioned stimulus - in this case, a "negative reinforcer" or "punishment". Thirty seconds of continuous exposure to a heat beam would incinerate the fly. A torque meter measured the fly's angular momentum around its vertical body axis - its yaw torque.
The description of the heat beam used in the experiments as a "punishment" raises the question of whether a fly's ability to be conditioned is grounded in its capacity to experience subjective mental states (e.g. pain and fear). Does the heat hurt the fly? And does the fly consciously fear an aversive stimulus? These questions will be further discussed in chapter 4. For the time being, it should be noted that avoidance behaviour alone cannot serve as an indicator of pain or fear: bacteria, whose behaviour is readily explicable using a goal-centred intentional stance, also avoid aversive stimuli.
Brembs (2000) tested fruit flies in four distinct "learning modes", where they were subjected to different combinations of classical and operant conditioning. Two modes were mono-dimensional tasks, where the fly had to form just one association. In operant conditioning, the association was between its behaviour (B) and a "punishing" unconditioned stimulus (US); in classical conditioning, between a visual pattern on the cylindrical panorama (CS) and the US. The other two modes were composite or multi-dimensional tasks, as the fly had the opportunity to form two associations: either B -> US and CS -> US (switch mode) or B -> CS and CS -> US (flight simulator mode).
Brembs (2000) measured the fly's ability to learn from pure operant conditioning by subjecting the fly to heat when it turned too far to the left or right. In this "yaw torque" mode of learning, the fly learned to avoid heat by restricting its yaw torque range, for example, to the left domain if straying into the right domain was punished. The fly thus formed an association between its behaviour (B) of initiating a right turn, and an unconditioned stimulus (US): heat. However, it had to learn this "blind" - i.e. without the help of any external guidance indicating whether it was flying inside or outside the permitted range. Heisenberg, Wolf and Brembs (2001) comment:
This is a remarkable feat, as ... in no natural situation would continuous turning to one side bring the fly permanently out of a heated zone (2001, p. 1).
In Brembs' classical conditioning experiments, a certain visual pattern (CS) on the cylindrical arena was followed by a punishment (US): the fly was subjected to heat. In this set-up, there was nothing the fly could do to avoid the punishment, but subsequent testing showed that it had learned to associate the pattern (CS) with the punishment (US).
In flight simulator mode (fs-mode), an attempt was made to realistically simulate the changes in the fly's visual field that the fly's turns would cause in free flight, except that the flight simulation was maintained at a constant speed at an undefined altitude. The cylindrical arena was decorated with four black T-shaped patterns of alternating orientation - two upright and two inverted - evenly spaced on the wall so that each quadrant had one T-shape. The arena's angular speed was made proportional to the fly's yaw torque, but the direction of rotation was opposite to that of the fly's turning. By adjusting its yaw torque, the fly was able to stabilise the arena and control the direction of its simulated flight (i.e. fly "straight" relative to the patterns on the wall). The fly was punished by heat (the US) when it flew into a forbidden quadrant, marked with an upright T, and it learned that quadrants associated with an inverted T were safe. In this learning mode, the fly was unable to form a direct association between its behaviour (B) and the heat (US), because the same behaviour (torque spikes) could either bring the fly into the heat or out of it, depending on which quadrant it was in. Instead, the fly had to form two associations. First, it had to associate its behaviour (B) of attempting to fly in the direction of a forbidden quadrant, with the CS (upright T) in that quadrant. Second, it had to associate the CS (upright T) with the aversive property of the US (heat), and learn that the former predicted the latter. This kind of learning is known as operant stimulus conditioning. It proved to be considerably more effective than either classical or pure operant conditioning alone. It was also a more natural way of learning. Whereas in the pure operant conditioning experiments, the fly had to restrict its range and continuously turn to one side to avoid the heat, in flight simulator mode, it simply had to keep its left and right yaw torque spikes within the safe zones.
In switch mode (sw-mode), the fly received two forms of feedback - heat (the US) and a change in the colour (from blue to green) or pattern (from an upright to an inverted T) of the panorama (the CS) - when its yaw torque exceeded the allowable range. For instance, if the fly's yaw torque value fell into the left domain, heat was switched on and the upright T pattern was placed in front; flying in the right domain heat switched the heat off and the arena was quickly rotated by 90 degrees, shifting the inverted T to the front. Thus the fly underwent parallel operant conditioning (a combination of operant and classical conditioning), where it not only had the opportunity to associate its behaviour (B) with a US (heat), but also had the chance to associate a particular display - a colour or pattern (CS) - with the US. Unlike flight simulator mode, the fly had to respond in a very artificial way to avoid the heat: it had to keep flying around in circles to avoid being fried. The requisite behaviour was thus the same as for pure operant conditioning. Brembs wryly comments:
I find it hard to imagine a situation where flying in clockwise circles gets a fly incinerated, but counterclockwise circles prevent that (personal email, 11 August 2003).
In other research (Brembs, 2003), it was shown that flies could stabilise a rotating cylindrical arena by modulating their thrust instead of their yaw torque. Flies learned after a few seconds to adjust their thrust to an arbitrary level (chosen by the experimenter) that corresponded to no rotation of the arena. Less thrust caused the arena to rotate one way (say, counter-clockwise), while more thrust caused it to rotate the other way (clockwise).
Summary of Brembs' research findings

In Brembs' experiments (2000), it turned out that classical conditioning was much less effective than operant training. Heisenberg, Wolf and Brembs (2001, p. 4) suggest that in classical training, the fly may have been distracted by searching for a behaviour that could control the temperature.
In Brembs' experiments (2000), the flies, despite having two forms of feedback, did not learn to restrict their yaw torque range significantly faster in sw-mode than in operant learning (yaw torque mode, where only B -> US associations could be formed). Brembs (2000, p. 26) attributed this negative result to the "artificiality" of the sw-mode set-up. (On the other hand, Heisenberg, Wolf and Brembs (2001, p. 5) found that sw-mode learning was more efficient than operant learning.)
Flies performed much better in fs-mode than in classical conditioning, where they were powerless to control their circumstances. "As expected, the more natural complex learning tasks are easier to solve than the more artificial single-association tasks" (Brembs, 2000, p. 30).
Generally, flies learned best in natural situations involving complex associations than in artificial set-ups with single-association tasks (pure operant conditioning or classical conditioning).
Classical conditioning and operant conditioning were shown to be independent learning processes.
It was also demonstrated that operant conditioning could assist (or boost) learning via classical conditioning, and that classical associations could be formed during operant training.
Flies were able to transfer colour or pattern preferences acquired during one mode of training (sw-mode) to another mode of learning (fs-mode).
It was shown that flies could remember patterns without heat reinforcement and compare them to other patterns later.
Additionally, the flies' ability to learn compound stimuli was investigated by using both colors and patterns as visual cues. It was shown for the first time that Drosophila could learn compound stimuli and recall the individual components independently and in similar proportions (Brembs, 2000, p. 31). Interestingly, it was discovered that "flies acquire, store and retrieve the two CSs 'colors' and 'patterns orientations' separately. They do not store them only as a compound" (Brembs, 2000, p. 30).
Higher-order associative learning in Drosophila melanogaster : a summary of research findings by Brembs and Heisenberg (2001)

Brembs (2000) and Brembs and Heisenberg (2001) searched for certain higher-order forms of associative learning that are commonly found in vertebrates undergoing associative learning: blocking, overshadowing, sensory pre-conditioning (SPC) and second-order conditioning (SOC). The two types of conditioned stimuli used (visual patterns and colors) allowed the researchers to study the effects of compound compound stimuli and, in particular, to investigate whether overshadowing, blocking, SOC and SPC could be observed in fruit flies. Confirmation of these phenomena in Drosophila would indicate that the same laws of learning apply to both fruit flies and vertebrates.
In blocking, reinforced exposure to conditioned stimulus A alone (i.e. exposure to A followed by a US), followed by reinforced exposure to a compound stimulus AB, prevents (or blocks) the animal from responding when stimulus B is presented alone, despite its prior association with the reinforcement. In overshadowing, a stimulus that can normally condition a response when presented as a CS on its own will acquire a much weaker association with the US when presented with another, stronger, stimulus (e.g., a more intense tone or light). Thus one stimulus 'overshadows' the other. Sensory preconditioning means that reinforcement of stimulus A after unreinforced exposure to a compound AB also leads to responses to stimulus B, while second-order conditioning means that reinforcement of stimulus A followed by unreinforced exposure to a compound AB also leads to responses to stimulus B.
Of the four forms of higher-order learning, the only strong effect was for sensory pre-conditioning. Second-order conditioning was very weak. Overshadowing was inferred, while bocking was not observed, despite repeated attempts to find it. The findings are presented in detail below. The conclusion drawn is that none of the phenomena necessitates a mentalistic interpretation of Drosophila's behaviour.
Blocking
Blocking was not observed in Drosophila melanogaster at the torque meter, despite careful efforts to detect it. Indeed, Brembs and Heisenberg (2001, p. 2855) reported that "We could find no unambiguous or undisputed evidence in the literature that invertebrates exhibit blocking." One salient difference between blocking experiments conducted with Drosophila and those carried out with vertebrates is the time-scale used for the training:
Whereas, in our experiments, training in the first phase of the experiment lasted for no longer than 8 min[utes], in the experiments on vertebrates it lasted for long periods, sometimes for a whole week. Vertebrates may use this extensive training to explore the situation and to generate memory templates with much higher reliability than can ever be obtained with our design. In the flight simulator, in particular, the fly with a single degree of behavioural freedom has little opportunity to explore the situation and to increase its level of 'orientedness'... In addition, 8 min[utes] in the life of a fly might well be as long as several days in the life of a rat or a pigeon. Perhaps blocking occurs only if the initial training has not only rendered the CS1 a certain or almost certain predictor of the US, but has, in addition, been stored in the memory reliably enough to render CS1 particularly difficult to extinguish during further training (Brembs and Heisenberg, 2001, p. 2854).
The alternative possibility is that invertebrates simply do not exhibit blocking. Brembs and Heisenberg (2001, p. 2855) speculate that invertebrates' memory templates are less reliable than those of vertebrates. For invertebrates, "[t]here is no reason not to remember a stimulus, even if it is only vaguely predictive for the US" (2001, p. 2855). On the other hand, vertebrates, with their larger, more complex brains, probably possess a superior ability to rapidly discern essential from redundant information, so they can afford to ignore (block) information about connections between redundant stimuli and a reinforcer (US) (Brembs, 2000, pp. 28-29). For them the costs of blocking outweigh the benefits.
Overshadowing
Overshadowing was not directly observed, but was inferred in Drosophila from the observation that patterns and colours were learned better if trained and tested alone than if trained and tested in a compound and then tested separately. In any case, "[o]vershadowing is a well-known phenomenon in classical (e.g. James and Wagner, 1980; Rauhut et al., 1999; Rubeling, 1993; Tennant and Bitterman, 1975) and operant (e.g. Farthing and Hearst, 1970; Miles and Jenkins, 1973) conditioning in vertebrates and invertebrates (e.g. Couvillon et al., 1996; Pelz et al., 1997; Smith, 1998)" (Brembs and Heisenberg, 2001, p. 2854).
Sensory pre-conditioning
Sensory pre-conditioning (SPC) was also verified in fruit flies. In sensory preconditioning, exposure to the compound (CS1+CS2) precedes training (CS1+US). "Hence, no extinction [the process of eliminating or reducing a conditioned response by not reinforcing it - V. T.] can occur between training and testing. Flies were exposed to 16 min[utes] of unreinforced flight in which flight directions were designated by compound stimuli consisting of colours and patterns (CS1+CS2). If, immediately afterwards, one of the stimuli is paired with heat (CS1+US), the other one (CS2) is regarded as a predictor of safe and dangerous flight orientations, respectively, in the subsequent test" (Brembs and Heisenberg, 2001, p. 2854). This experiment proved that CS-US pairings are not necessary for a CS to accrue associative strength.
Second-order conditioning
The second-order conditioning (SOC) effect observed was much smaller than the sensory prec-conditioning effect. The authors speculated that this may have been because in SOC, the compound (CS1+CS2) is presented after the initial training with the single stimulus (CS1+US). In the intervening period, the conditioned response observed in the flies may have time to attenuate: "the presentation of the compound without heat after the conditioning may lead to extinction of the learned association attenuating the CS1-US association (extinction)" (Brembs and Heisenberg, 2001, p. 2853).
The authors' conclusion was cautiously up-beat:
As in vertebrates, associative learning in invertebrates requires complex processing of sensory stimuli during memory acquisition. Further research is needed to determine the extent to which these processes are shared across phyla (Brembs and Heisenberg, 2001, p. 2861).
Summarising, Brembs proposes that Drosophila uses a "sophisticated, asymmetric set of rules ... [in] guiding its selection which of the predictors present in a composite learning situation are to be stored in memory for later use" (2000, p. 30).
The term "rules" might suggest that Drosophila is engaging in some kind of cognitive processing. However, in a personal email (22 December 2002), Brembs repudiated such an interpretation:
You may have noticed that I try to avoid the use of the word "cognitive". For my purposes, the distinction into cognitive and non-cognitive has no heuristic value... I personally keep a tally of tasks (in my head) of what different animals have or haven't shown to be able to successfully complete. Eventually, I want to find out how the brain solves these tasks. The question of what parts of the brain are contributing how will be answered then and the question how 'cognitive' the involved processes are, will be redundant...
Some of the above mentioned phenomena have warranted explanations that include cognition-like concepts of attention or expectation and prediction - which we discussed in the case study relating to worms, in connection with blocking. However, alternative non-cognitive interpretations are possible. Although it has been shown that some insects (e.g. Drosophila) are capable of complex learning tasks and exhibit some higher order forms of associative learning that are found in vertebrates, it has not yet been established that a first-person intentional stance or an agent-centred stance is required to explain these feats.
To sum up: despite the similarities in higher-order associative learning between insects and vertebrates do not, it has not been shown that higher-order associative learning in insects requires a mentalistic explanation. There is no reason to believe that a mind-neutral goal-centred intentional stance cannot account for the behaviour observed.
However, in Part C (Section 1) of chapter two, I argue that operant conditioning in Drosophila does indeed contain a number of features which, when taken together, warrant the attribution of agency and mental states (beliefs and desires) to insects.
2. Spatial navigation: the status of cognitive maps in insects, according to recent research

Menzel, Brandt, Gumbert, Komischke and Kunze (2000) claim that some insects e.g. (honeybees) possess two spatial reference systems:
(i) an egocentric (observer-centred) system, also known as specialised route memory (SRM), which makes use of path integration and a sequence of landmarks; and
(ii) an allocentric (world-centred) system, which combines multiple views and movements in a common frame of reference.
The authors refer to the latter system as a general landscape memory (GLM). They suggest it is learned by bees during their reconnoitring flights around a new hive, and is only activated when the SRM cannot be used. They speculate about how the GLM is implemented at the neural level: it may simply list each landmark with its vector to the hive, or it may include multiple sequential views of landmarks, acquired during reconnaissance flights, or it may store the landmarks in a spatial memory, with a graph structure.
Giurfa and Capaldi (1999, p. 237) define a cognitive map as "a form of spatial memory, in which the geometrical relationships between defined points in space are preserved", from which it follows that "a cognitive map should allow novel short-cutting to occur".
Experiments designed to test novel short-cutting in bees produced conflicting results during the 1980s and 1990s. Recently, studies have shown that honeybees can use novel routes (Menzel, Brandt, Gumbert, Komischke and Kunze, 2000). Tests were performed near a prominent landmark (a steep, isolated hill). Foraging bees were captured at the training station and released at different sites, up to 180 degrees from their original bearings. Nevertheless, the bees generally managed to re-orient themselves towards the hive. Bees released at much greater distances, which they had never visited before, were unable to do this.
Novel short-cutting has been claimed by other researchers (e.g. Giurfa and Menzel, 2003), who summarise their findings as follows:
Furthermore, route-trained bees carrying a trans-ponder enabling harmonic radar to locate them were captured and released at a novel site, either when leaving the feeder to return to the hive, or when arriving empty at the feeder... Both groups of bees first flew their usual vector when released at a novel site, but then headed towards the hive after a phase of circling flights. Again, beacon orientation and navigation according to landscape features were excluded. Most importantly, bees sometimes also decided to fly back to the feeder first rather than directly to the hive. These and additional experiments can be explained by assuming that during their orientation flights, bees learned different locations in their surroundings and attached to the landmarks characterizing these locations local vectors pointing towards the hive...[T]he fact that bees foraging at a distant and constant feeder could decide to fly first back to the feeder rather than directly to the hive indicates a form of spatial memory in which some geometrical relationships between defined points in space are preserved, in agreement with Tolman's seminal paper...
However, alternative explanations have been proposed, and the existence of cognitive maps or even a GLM is disputed (Collett and Collett, 2002; Harrison and Schunn, 2003; Giurfa and Capaldi, 1999).
[A]ll around the nest, different landmark constellations might be linked to different flight vectors. In this way, bees might find their way home from any novel release site within this area, providing that it lies between two identifiable sites that give rise to different, established flight vectors. This type of vector map is different from a cognitive map... (Giurfa and Capaldi, 1999, p. 241).
Recently, Harrison and Schunn (2003) have tried to establish that egocentric processing is computationally cheaper than allocentric or exocentric processing, and that the cognitive map hypothesis is redundant, even for rats.
3. Tool use in cephalopods
Taxonomy and comparative anatomy
Cephalopods are a class of the phylum Mollusca (molluscs) and are therefore related to bivalves scallops, oysters, clams, snails and slugs, tusk shells and chitons. Cephalopods include the pelagic, shelled nautiloids and the coeleoids (cuttlefish, squid and octopods, the group to which octopuses belong). (Authorities agree that the plural of octopus cannot be octopi, as the word is Greek, not Latin.)
The design of a mollusc's nervous system is quite different from that of a vertebrate, despite fundamental similarities at the neuronal level:
The vertebrate central nervous system comprises one main nerve cord that has swollen at one end to create a brain. Most molluscs, on the other hand, have dual nerve cords running like a set of railway tracks along the length of the body (Hamilton, 1997, p. 32).
Among the molluscs, there is an enormous degree of variability in the complexity of the nervous system. Cephalopods are renowned for their large brains, while other molluscs (e.g. bivalves) lack even a head, let alone a proper brain. Most molluscs have a relatively "simple" central nervous system, with five or six pairs of ganglia.
In the cephalopods alone among the molluscs, evolution has also constructed a brain. It has greatly expanded the forwardmost pairs of ganglia and moved them closer together to create a tightly packed mass of lobes that lies between the eyes and encircles the oesophagus (Hamilton, 1997, p. 32).
The brain-to-body weight ratios of cephalopods exceed those of other invertebrates, as well as most fish and reptiles. Additionally, their brains are anatomically complex. However, mammals and birds far outstrip cephalopods in the complexity of their brains (Anderson and Wood, 2001; Hamilton, 1997).
In contrast with molluscs such as clams and oysters, which are passive filter feeders, cephalpods live in a challenging environment, where they have to hunt down mobile prey and avoid predators. They have sophisticated sense organs, a complex rapid movement system, an ability to rapidly change colour and (in the case of cuttlefish and squid) a wide range of social signals (Broom, 2001).
What do cephalopods think with?
So far, we have assumed that cephalopods think with their brains.
Most cephalopods have very flexible limbs, with unlimited degrees of freedom. Scientists have recently discovered that octopuses control the movement of their limbs by using a decentralised system, where most of the fine-tuning occurs in the limb itself:
...[A]n octopus moves its arms simply by sending a "move" command from its brain to its arm and telling it how far to move.The arm does the rest, controlling its own movement as it extends.
"There appears to be an underlying motor program... which does not require continuous central control," the researchers write (Noble, 2001).
I suggest that there is no inherent reason why intelligence should be tied to a brain alone. We should be open to the possibility of creatures who also think with their arms, especially when "each arm is controlled by an elaborate nervous system consisting of around 50 million neurons" (Noble, 2001).
The learning abilities and adaptive behaviour of cephalopods compare favourably with those of insects and some vertebrates. The following discussion focuses principally on the well-studied common octopus, Octopus vulgaris.
Spatial learning
Cephalopods find their way around by remembering landmarks, as well as the distances they have travelled:
Cephalopods are certainly adept at navigation. Mather and a team of volunteers have mapped the travels of fist-sized O[ctopus] vulgaris as they forage off the coast of Bermuda. The animals venture from their dens on complicated trips lasting up to three hours, and return by different, more direct routes. Although O. vulgaris usually ends up no more than 9 metres from home, other species of octopus can find their dens after journeys of up to 120 metres - over a landscape that easily disorients human scuba divers (Hamilton, 1997, p. 35).
Like some insects, octopuses can navigate flexibly:
A series of disruptions of the foraging trail showed that they could make detours and suggested they were using vision to follow prominent features of the landscape of the rocky bottom (Mather and Anderson, 1998).
The ability of insects to navigate using landmarks has already been identified as indicative of cognitive mental states (see Part C), irrespective of whether they use allocentric cognitive maps.
Additionally, laboratory tests have shown that octopuses are fast learners that adapt quickly to reversals. They can rapidly learn the location of an escape burrow in an arena and retain this information for a week. When the burrow location is rotated 180 degrees, they display relearning (Langley, 2002).
Octopuses can navigate simple mazes. Lauren Hvorecny and Jessica Grudowski are currently researching learning in octopuses, to determine if they can solve a conditional discrimination maze problem (in maze configuration A, go to hole A; in maze configuration B, go to hole B). The results are still being analysed. The only invertebrate that has demonstrated this type of complex learning is the honeybee.
Conceptual Learning
Experiments on octopuses performed by J. Z. Young in the 1950s and 1960s showed that they can learn to distinguish between shapes, orientations, sizes and degrees of brightness:
In one experiment, Young trained octopuses to select between large and small squares, horizontal and vertical stripes, and black and white circles. He found that the animals could retain all three preferences at once (Hamilton, 1997, p. 34).
However, discrimination is not the same as conceptualisation. Evidence for the latter would be more convincing if it could be demonstrated that octopuses, like honey bees, were able to make distinctions at a more abstract level - e.g. between symmetrical and asymmetrical, or same and different. Research to date on whether octopuses get "the oddity concept" is inconclusive (Mather, personal email, 8 September 2003).
The ability to change bodily appearance: camouflage, mimicry, signaling and deceit
Cephalopods have an ability to change their appearance which is unrivalled among other animals, thanks to the presence of thousands or even millions of rapidly migrating chromatophores (multi-celled organs containing pigment sacs of various colours) in their skin, which allow them to blend in with their background. It takes less than a second for cephalopods to adopt a new colour pattern, as the process is controlled by the brain through nervous impulses to the muscles. Additionally, cephalopods have soft, flexible bodies and muscles that allow them to change the texture of their skin (Hamilton, 1997; Langley, 2002; Milius, 2001).
Cephalopods use their ability to change their colour patterns and skin texture for various purposes. To avoid being eaten by a predator, they may either blend in with their backgound, or mimic animals that taste bad to the predator, or even mimic animals which feed on the predator (Hamilton, 1997; Langley, 2002; Milius, 2001).

Indonesian octopus (left column) mimics a banded
sole (top right) and a banded sea snake (bottom
right). Courtesy M. Norman and R. Steene.
An outstanding example is the newly discovered "mimic octopus" of Indonesia, described recently by Norman, Finn and Tregenza (2001). It is able to forage in broad daylight, thanks to its ability to impersonate toxic or predatory species as diverse as sea snakes and fish. The octopus also changes its postures and body movements to mimic its models:
Sometimes, the octopus fled with its arms aligned in a flattened, striped oval, looking much like a common poisonous flatfish. On four occasions when damselfish pestered an octopus, Norman saw it poke six of its legs down a burrow and spread the other two. They sported bands and waved gently, resembling the sea snakes that prey on damselfish.When Norman saw a mimic octopus chugging along well above the seafloor, extended arms colored in stripes, he thought of the sunburst of striped, poisonous spines that lionfish flare (Milius, 2001).
Cephalopods also change their colour patterns and texture to camouflage themselves while hunting prey, to signal (or disguise) their intentions during courtship, and to deceive or ward off attacks by rival males (Hamilton, 1997).
The Caribbean reef squid affords a spectacular example of this behaviour:
[It] has at least 35 patterns in addition to its almost magical ability to blend in with its background. It can flash a different display on each side of its body when positioned between a potential mate, which sees a uniform light grey, and a rival male, which sees tiger striping called the "intense zebra display". If the positions change, so do the patterns (Hamilton, 1997, p. 33).
Similarly, male cuttlefish adopt female colouring, patterns, and shape, to gain access to females guarded by larger rivals (Scigliano, 2003).
The behaviour described here can easily be interpreted in intentional terms: mimicry, disguise, strategic planning and deception. A useful question to ask might be: do we need to adopt an agent-centred stance in order to account for the behaviour? To answer this question, we need to do three things. First, we need to discover what causes these colour and texture changes in cephalopods. Are they triggered by simple reflexes, or is there at least some scope for fine motor control, as we observed in Drosophila, which would allow us to speak of agency here? Unfortunately, the sheer rapidity of the changes makes it difficult to investigate their etiology.
Second, we have to find out whether cephalopods are physically capable of controlling (i.e. fine-tuning) their color and texture changes. In my discussion of agency in Drosophila, I suggested that fine motor control required an interaction between an animal's feedforward and feedback mechanisms, and that efferent copy played a vital role. Certainly, the body movements of the Indonesian mimic octopus appear fine-tuned to the circumstances.
Colour changes in cephalopods are more problematic. Although they are directed by the brain via the nervous system, no muscular movements appear to be involved. Can there be trying wihout muscular activity? It is hard to see how we can speak of a cephalopod as trying to turn black unless it can compare its current colour with that of its surroundings and adjust its bodily movements accordingly. On the other hand, processes which are involuntary in vertebrate nervous systems may not be so in cephalopods.
Third, we need to investigate whether the behaviour observed is a fixed action pattern or whether it is truly flexible, as defined in this thesis. For instance, is there any evidence of learning? Do young cephalopods display a "learning curve" when camouflaging their appearance, mimicking other animals or signaling to mates? And can they learn by watching their peers? (The answer to the last question is probably negative.)
Flexible behaviour in octopuses?
Mather and Anderson (2000) describe how octopuses will use a variety of techniques to open a clam shell, switching readily from one to another in the event of failure. Giant Pacific octopuses switch strategies to open different shellfish - smashing thin mussels, prying open clams, and drilling tougher-shelled clams. When clams were wired shut with stainless steel wire, the octopuses couldn't pull them apart, so they switched to drilling and chipping. The authors comment:
They were intelligently adapting the penetration technique to the clam species presented and the situation in which they were placed.
The above interpretation is reasonable. Unfortunately, the range of behaviours involved here is too narrow to decide whether the octopuses were acting intentionally or in a hit-and-miss fashion.
Observational learning in cephalopods?
Fiorito and Scotto (1992) reported that an octopus in a research laboratory in Naples learned to choose a red ball instead of a white one, simply by watching another octopus. (Actually, octopuses prefer red over white, but the opposite preference has also been induced in recent experiments.) The discovery of observational learning, if confirmed, would be remarkable, as octopuses are short-lived, solitary creatures that usually meet only to copulate, and as even some mammals are incapable of this learning feat (Hamilton, 1997). However, other researchers, including Jean Boal, have tried without success to replicate the results (Mather, personal email, 8 September 2003). Commenting on the original experiment, Woods (2003) writes:
A critique by Biederman and Davey of the Fiorito and Scotto experiment can be found in Science vol 259 (March 12, 1993). The critique questions: if the observational octopuses attacked the ball more often since it was a familiar item (i.e. octopuses are hesitant to attack novel stimuli), [and] if observational learning or rapid imitation occurred (what was the role of the stimuli and the role of the demonstrator octopus - why were these not controlled for?). I should mention that Fiorito defends the experiment in the same issue of Science. The bottom line, at least in my mind, is that the Fiorito and Scotto experiment failed to prove observational learning since other factors were not controlled for. I certainly would not rule out the possibility of observational learning in cephalopods - after all they are the most advanced invertebrates. On the other hand, octopuses are not very social so there may not be much of a chance for them to evolve the ability to learn by observing other octopuses.
On methodological grounds alone, it would be imprudent to ascribe observational learning to cephalopods. There is another reasons to question the cognitive interpretation of the octopuses' behaviour: learning can also be triggered by events that convey no technical (means-end) information, suggesting that the skills are latent within the octopus and not learned.
Late last year at Woods Hole, Boal, Hanlon and graduate student Kim Wittenberg allowed animals to observe trained cuttlefish attack and eat a crab, and then compared their performance in the same situation with a naive animal. The observers did learn more quickly how to hunt down a crab. But they also hunted better if they had previously seen only a crab without a predation event, or even if they had simply smelt that a crab was kept hidden behind a partition. 'If smelling a crab means you perform better than if you hadn't smelled one before, and watching a predation event is no better than simply smelling a crab,' says Boal, 'then we're talking [about] some kind of releaser of an innate behavior' (Hamilton, 1997, p. 35).
Play in octopuses
Mather and Anderson (1999) define play as "activity having no immediate benefits and structurally including repetitive or exaggerated actions that may be out of sequence or disordered", and reported observing some octopuses playing with objects. Scigliano (2003) describes their experiment:
Anderson tested for play by presenting eight giant Pacific octopuses with floating pill bottles in varying colors and textures twice a day for five days. Six octopuses examined the bottles and lost interest, but two blew them repeatedly into their tanks' jets. One propelled a bottle at an angle so it circled the tank; the other shot it so it rebounded quickly and on three occasions shot it back at least 20 times, as if it were bouncing a ball.
However, Boal questions the authors' interpretation, and suggests that the behaviour may reflect boredom (like a cat pacing), rather than creativity. More recently, another researcher, Ulrike Griebel, offered common octopuses a variety of objects, from Lego assemblies to floating bottles on strings. Some octopuses took toys into their nests and toted them along while fetching food. Griebel suggests that this "might be an early stage of object play" (Scigliano, 2003).
Mather and Anderson (2000) argue that "[p]lay involves the detachment of actions from their primary context, and such flexibility is both a basis and a sign of intelligence, whether it be shown in a person or a fish or an octopus." The key insight here is that the player self-selects a new goal and performs the actions to achieve this goal rather than the natural end of the behaviour. Genuine play is therefore cognitive.
The upshot of our overview is that although we cannot be as certain as for insects, cephalopods also appear to be creatures with minds of their own, possibly rivalling those of some vertebrates.
4. Social learning in fish

In my model of social agency in fish, I outlined a proposed set of sufficient criteria for agency in a social context, looked at the rationale for social learning, and noted its widespread occurrence across vertebrate species. Evidence that at least some species of fish satisfy my criteria for social agency is provided below. I should note that to date, I have not been able to establish whether any particular species meets all of the criteria, although the evidence for cleaner fish, sticklebacks and guppies looks impressive.
Behaviour modelled on that of a knowledgeable individual
Individuals (especially juveniles) learn to model their behaviour on that of experienced adults, mainly by accompanying them and observing how they behave (Bshary, Wickler and Fricke, 2002).
Sensory discrimination between individuals and/or categories of individuals
There is abundant evidence in the literature of individual recognition in fish:
Individual recognition based primarily on optical cues ... has been demonstrated experimentally in a variety of species... There is even evidence that in damselfish, individuals can recognise one another on purely acoustical cues... In summary, individual recognition can safely be assumed to be widespread across fish families (Bshary, Wickler and Fricke, 2002).
In addition to individual recognition, cleaning symbiosis provides an example of a case where the ability to categorise individuals on the basis of their observed characteristics is especially useful:
In cleaning symbiosis, so-called client fish trade the removal of parasites and dead or infected tissue against an easy meal for so-called cleaner fish... Cleaning symbiosis is particularly promising for comparative studies as cleaner fish are found in many different fish families and can differ markedly in the degree to which they depend on interactions with clients for their diet... Full-time cleaners like the cleaner wrasse (Labroides dimidiatus) may have about 2,300 interactions per day with clients belonging to over 100 different species... There is strong evidence that cleaners can categorise their 100-or-so client species into resident species that have access to their local cleaner only, due to their small territory or home range, and other species that have home ranges that cover several cleaning stations. As predicted by biological market theory (Noe et al. 1991), clients with choice options between cleaners almost invariably have priority of access over clients without choice at cleaning stations (Bshary, Wickler and Fricke, 2002).
Memory for individuals and their track record
The ability to remember individuals over long periods of time is of fundamental importance for social learning. Bshary, Wickler and Fricke (2002) cite evidence that an anemonefish can recognise an individual that it has not seen for 30 days. (So much for the myth that fish have only a 3-second memory!)
According to Bshary, Wickler and Fricke (2002), some fish can also monitor changes in the status of individuals and track relationships within their groups.
Bshary, Wickler and Fricke (2002) describe experiments showing that some fish species are capable of engaging in book-keeping (remembering their partners' behaviour during past interations) with several partners at once:
The most famous example of co-operation in fish is probably the inspection of nearby predators by one or several fish that leave the relative safety of their school to do so (Pitcher et al. 1986). During inspection, pairs of sticklebacks, Gasterosteus aculeatus, and guppies, Poecilia reticulata, among others, approach the predator in alternating moves. A series of experiments led to the conclusion that these fish solve a so-called "prisoner's dilemma" (Luce and Raiffa 1957). In a prisoner's dilemma, two players have the option of either co-operating with or cheating their partner. Cheating the partner yields a higher benefit than co-operation irrespective of what the partner does, but if both partners co-operate then they receive a higher benefit than if both cheat, hence the dilemma. Milinski (1987) and Dugatkin (1988) proposed that fish solve the prisoner's dilemma by playing a "tit-for-tat" strategy, which states that a player starts co-operatively and does in all further rounds what the partner did in the previous round (Axelrod and Hamilton 1981). This interpretation is not yet entirely resolved (see review in Dugatkin 1997) but discussions about the interpretation led to a few experiments with very interesting additional results. Milinski et al. (1990a) could show that individual sticklebacks prefer specific partners to others, which implies that school members recognise each other. In addition, partners build up trust in each other during repeated inspections, that is, they hesitate less in approaching a predator when accompanied by a partner that co-operated in the past (Milinski et al. 1990b). Similar results have been found in guppies (see review in Dugatkin 1997). These data imply that these fish species are capable of book-keeping (remembering their partners' behaviour during past interactions) with several partners simultaneously (2002).
Observational learning of new practices
Juvenile fish learn what to eat by observing conspecifics:
There is some evidence that young fish learn what to eat by observing adults. Fish definitely learn horizontally from conspecifics what to eat under lab conditions. Templeton ... found that juvenile rock bass ... that saw a trained conspecific eating a novel food item would readily consume that food later when, alone, they were tested for the first time. Without prior observations, these juveniles did not attack the prey... (Bshary, Wickler and Fricke, 2002).
Hatchery-reared Atlantic salmon acquire new kinds of feeding behaviour and learn to target new kinds of prey, simply by observing knowledgeable conspecifics. Salmon raised in hatcheries tend to prefer taking prey from the surface, because of long-term conditioning in the hatchery environment. This can cause high mortality rates from starvation when they are released into the wild, because their choice of prey near the surface is restricted and energetically costly. However, after six days of watching "demonstrators" through a clear perspex partition, naive salmon changed their feeding habits and were able to feed from the bottom (Brown, Markula and Laland, 2003).
Social enhancement of foraging has been reported in species as different as salmon, rock bass, Alaska pollack and brown trout (Brown and Laland, 2003).
Fish can also learn novel techniques for obtaining food from observation of knowledgeable conspecifics. Juvenile European sea bass learned to press a lever to get food, simply by watching other fish that had been previously trained to do this (Brown and Laland, 2003).
Learning (group traditions)
Schools of fish have their own "traditions" relating to their choice of sites for resting sites, migration routes and food sources, and this knowledge is transmitted through social learning (Bshary, Wickler and Fricke, 2002). For instance, juvenile French grunts learn the migration route from their resting grounds to feeding sites by following older individuals, and bluehead wrasse have prefrred mating sites that stay the same over many generations (Brown and Laland 2003).
Bshary, Wickler and Fricke describe the mechanism by which traditions are perpetuated in guppies:
Laland and Williams ... conducted laboratory experiments and showed experimentally that guppies learn the way to hidden food sourced from knowledgeable conspecifics. The conspecifics had been trained to use only one of two ways to the food source. Naive fish were added and learned the way to the food source by schooling with the others. Members of the original school could be replaced successively and the school still preferentially took the originally learned way to the food source. The fish thus built up a tradition. Using principally the same experimental set up, Laland and Williams ... went one step further and showed that even maladaptive behaviour can spread through a population due to social learning. In their study, a longer and therefore more costly way to a foraging site was still preferred over a short way 3 days after all original trainers had been removed" (Bshary, Wickler and Fricke, 2002).
Innate goals
Brown and Laland (2003) mention four general categories of goals, in relation to which social learning is known to take place amongst fish: predator avoidance; migration and orientation; foraging for food; and mate choice. There is an ever-growing body of evidence that juvenile fish engage in extensive social learning of skills relating to all of these goals (Laland, Brown and Krause, 2003).
Fine-tuning (controlled, modulated activity):
Individuals carefully tailor their own social behaviour towards an individual, in accordance with their observations of that individual's past interactions with other individuals.
Male Siamese fighting fish ... monitor aggressive interactions between neighbouring conspecifics and use the information on relative fighting ability in subsequent aggressive interactions with the males they have observed...(Brown and Laland, 2003, p. 285).
Individuals also adjust their behaviour towards a specific individual on the basis of their own previous interactions with that individual - a practice known as book-keeping. Cleaner fish engage in book-keeping: they provide better than average service to dissatisfied clients that "punished" (aggressively chased) them during their last interaction. As Bshary, Wickler and Fricke (2002) point out, punishment can only work if there is individual recognition. This means that cleaner fish must be able to keep track of the behaviour of each their clients (up to 100 individuals!), and modulate their behaviour towards each of them.
Cleaner fish also provide tactile stimulation to predatory clients, possibly as a form of pre-conflict management, or towards clients it has cheated in the past (Bshary, Wickler and Fricke, 2002).
Additionally, cleaner fish behave much more attentively (or "altruistically") towards their clients if they are being watched by bystanders who have the option of switching to another cleaning station. The reason is that an observer will copy the behaviour of the previous client, and either invite for inspection if it witnessed a positive interaction, or flee the approaching cleaner if it saw the last client run away as well. The true rationale for cleaner "altruism" is a selfish one: the opportunity to recruit a new customer and get access to more food (Bshary, Wickler and Fricke, 2002).
Internal representations
The mechanisms by which fish represent their social interactions with other individuals are not known, but fish are certainly able to form internal representations of the status and fighting ability of other individuals in their group, as well as the reliability of former partners (Bshary, Wickler and Fricke, 2002). Presumably, when copying the goal-oriented behaviour of a knowledgeable individual, they must be able to represent the activity of following the role model's example as a means of attaining its own ends, which are (qualitatively) the same as its own. Alternatively, in simpler cases (e.g finding hidden food by following a knowledgeable individual), the observer may simply represent the model itself as a kind of "moving signpost" pointing to its goal (i.e. the model itself is viewed as a means to the individual's end).
Self-correction
Fish are certainly capable of altering their social behaviour when their expectations of another individual are disappointed. As we saw above, Bshary, Wickler and Fricke (2002) cited evidence that sticklebacks and guppies adopt a tit-for-tat strategy towards their partners: a partner that fails to co-operate is punished the next time round. Cleaner fish who cheat their clients by removing extra food (healthy tissue) as well as dead or infected tissue, are "punished" (chased aggressively).
Other evidence for intentional agency in fish
According to Bshary et al. (2002):
there is an array of behaviours found in a variety of fish families (categorisation, cheating, punishment, manipulation of individuals and altruism) which are usually thought of as unique to primates;there are instances of interspecific cooperative hunting between giant moray eels and red sea coral groupers;
co-operative hunting between conspecific predators is widespread in fish, and different individuals play different roles;
some fish appear to be able to use cognitive maps of their environment. For instance, inter-tidal gobies acquire an effective memory of the topography of its home pool as well as that of surrounding pools, because at low tide, it often has to jump into these pools without being able to see where it is going. Other fish appear to use landmarks for homing;
some fish use advanced foraging techniques - e.g. removing obstacles to reach hidden prey, and using their spatial intelligence to gain access to prey;
a few fish are capable of tool-using behaviour in the strict sense of the word (Beck, 1980), where an animal directly handles an object in order to obtain a goal. South American cichlids are a case in point;
some fish also build complex nests and bowers (Laland, Brown and Krause, 2003).
Finally, contrary to claims by Varner (1998), fish are indeed capable of progressive adjustments in multiple reversal trials - as long as olfactory stimuli are used (Mackintosh and Cauty, 1971, cited by Wakelin, 2003). Earlier, we examined arguments that creatures which show improvements in serial reversal learning were capable of meta-learning, insofar as they had to develop primitive hypotheses about changes in their surroundings. We tentatively concluded that this was the most reasonable interpretation of the experimental evidence, and that the behaviour should be described using an agent-centred intentional stance.
Although learning in the broadest sense (i.e. the acquisition of new skills) may not necessarily require mental states, there is no doubt that meta-learning ("learning to learn") is a critical capacity that presupposes an ability to evaluate and correct one's actions. A creature with this ability would qualify as what Dennett (1997, p. 112ff.) calls a "Popperian creature". Such a creature is one level above a Skinnerian creature, which is capable of learning from its trial-and-error mistakes and successes, and can associate information about one kind of event with information about another kind. A Skinnerian creature may stumble upon a "smart move", but it cannot predict what works and what does not. Its first move may be a fatal one, if it is unlucky.
A "Popperian creature" can avoid such an outcome, because it can foresee the consequences of its actions in the "inner environment" of its imagination, which lets the creature manipulate information in its memory, about its external environment. In this inner environment, try-outs or simulations can be executed without harming the animal, allowing it to select the best course of action and make a smart first move in its external ("real") environment. The advantage of foresight is that it "permits our hypotheses to die in our stead", as Popper put it (Dennett, 1997, p. 116). The creature can make a smart first move, because it can think about smart moves.
Varner (1998), following Bitterman (1965), has suggested an experimental way to identify meta-learning in animals and resolve the question of whether they have mental states. He has argued that if they are genuinely learning, (and not merely mechanically associating), they should be forming hypotheses about the changes in their environment. He has proposed that reversal tests offer a good way to test animals' abilities. Multiple reversal tests involve repeatedly reversing the reward pattern in simple learning experiments. For instance, a rat is first presented with two levers and rewarded for pressing the left lever instead of the right. When the rat has learned to press the left lever all the time, the reward pattern is reversed. Once the rat has learned the new reward pattern, it is reversed again, and so on. Varner suggests that if an animal shows no improvements in the time it takes to adjust to subsequent reversals, that suggests an inflexible, non-cognitive mechanism is governing its behaviour. By contrast, Bitterman predicted that an animal that can form hypotheses should take longer to learn the new pattern the first time it is reversed, but should adjust more and more rapidly to subsequent reversals, as it learns to quickly revise its expectations.
Varner's proposal invites two questions. First, is rapid reversal learning a sign of intelligence? Second, does progressive improvement in multiple reversal tests indicate the presence of mental states?
The ability to adapt rapidly to changes sounds like a mind-like feature. However, the consensus from animal behaviourists is that it need not be so. According to Ben-Shahar (personal email communication, 19 August 2003), the rapid reversal learning of honey bees surpasses even that of pigeons and rats. However, Ben-Shahar cautions against the use of reversal learning per se as a measure of intelligence in animals, as the rapid reversal learning appears to be an adaptive trait for some animals, and adaptive behaviour is not necessarily intelligent:
We can formulate the following negative conclusion:
The second and more interesting question is whether the existence of progressive adjustment in multiple reversal learning trials indicates intelligence.
The ability to improve in multiple reversal learning trials is readily explained by the hypothesis that the animal is forming a hypothesis about changes in its environment. I have not been able to find a non-cognitive explanation as to why such improvement might occur. Certainly, the fact that the cognitive explanation makes a highly specific prediction (that the animal should take longer to learn the new pattern the first time it is reversed), which has been experimentally confirmed, tends to bear out a mentalistic interpretation. It should be borne in mind, however, that even if the behaviour cannot be accounted for in terms of associative learning, that does not necessarily make it cognitive.
Even if progressive adjustment shows that an animal has mental states, that does not necessarily make it a Popperian creature. The ability to formulate primitive hypotheses need not imply the ability to foresee the consequences of one's actions in the "inner environment" of one's imagination.
Basing his arguments on research by Morton Bitterman (1965), Varner has claimed (1998, p. 32) that progressive adjustment in multiple reversal learning trials is found only in reptiles, birds and mammals. Since then, it has become apparent that fish (Wakelin, 2003) and honeybees (Komischke, Giurfa, Lachnit and Malun, 2002), are also capable of this kind of learning. Komischke, Giurfa, Lachnit and Malun (2002) compared the responses of bees that had experienced reversals with those of bees that had not experienced such reversals when both were confronted with a new reversal situation. They found that bees that had experienced three previous reversals were better in solving the final reversal task than bees with no previous reversal experience. They also showed that one reversal learning trial was enough for bees to perform better in the final reversal task.
The evidence to date from serial reversal learning suggests that honeybees, at least, are capable of learning to learn. This ability may turn out to be widespread among insects, but very little research has been done with most groups of insects. Brembs claims that serial reversal learning in insects is not confined to honeybees:
However, neither Brembs nor Drosophila researcher Josh Dubnau was able to supply a reference to serial reversal learning by Drosophila melanogaster in the published literature. Dubnau admitted that "not many people have looked carefully at reversal learning in flies" (personal email communication, 14 August 2003).
The evidence from serial reversal learning is thus of limited value. At most, it suggests that honeybees are capable of meta-learning, while saying nothing about other insects.
Concept formation is another area in which meta-learning can be shown to take place in insects. Tests by Gould and Gould (1988) showed that bees could learn to recognise and distinguish human letters, regardless of size, colour, position or font. Giurfa, Eichmann and Menzel (1996) trained foragers to associate symmetrical shapes with food. Asymmetrical shapes were not rewarded. (In another test, asymmetrical shapes were rewarded while symmetrical ones were not.) By the seventh visit, the bees could choose a correct novel stimulus over an incorrect one. Gould argues that this kind of learning differs from associative tasks:
The main difference is that honey bees are much quicker at deciphering what the experimenter wants than are pigeons and other standard laboratory animals (Gould, 2002, pp. 43-44, italics mine).
I would agree with Gould's claim that the only satisfactory explanation for the sudden improvement in the bees' performance is a cognitive one: the bees formed a generalised notion of a symmetrical (or asymmetrical) object. Prior associative learning of rewarded and unrewarded stimuli cannot explain their performance, as (a) the relevant property was not a low-level property such as "red" that they were hard-wired to recognise, and (b) the bees had to transfer their discriminatory abilities to novel stimuli. This requires "a capacity to detect and generalize symmetry or asymmetry" (Giurfa, Eichmann and Menzel, 1996, p. 458). It was also shown experimentally that bees have an innate preference for symmetrical shapes, but this in no way undermines a mentalistic explanation of their performance. The existence of a pattern preference in honeybees does not explain how the concept "asymmetrical" is acquired, or how bees suddenly "figure out" what to look for in novel stimuli, after several trials. These facts can only be accounted for by supposing that the bees were trying to learn what they had to do in order to obtain their reward consistently, and finally managed to form a general concept of what the rewards had in common. The fact that trained bees tend to pay more attention to symmetrical rather than asymmetrical stimuli simply shows that they can form some concepts (e.g. "symmetrical") more easily than others ("asymmetrical"). For that matter, as Gould (2002, p. 44) points out, human infants display the same innate preference.
Other research has shown that bees can discriminate vertical from horizontal stripes, and apply this distinction to novel stimuli showing the same patterns. These experiments satisfy the standard requirements for categorisation: (i) a variety of stimuli sharing some common feature are rewarded, rather than an individual stimulus; and (ii) the animals can transfer the concept to novel stimuli (Menzel and Giurfa, 2001, p. 66).
Is there a hidden bias in the search for conceptual learning? How do we form concepts for smells?
Incidentally, I would suggest that there is a hidden bias in the search for conceptual learning, which has so far impeded our search for this kind of learning in "simpler" animals such as worms. From a cognitive perspective, a concept, properly speaking, is more than a mere category such as "red", which an animal could be neurally hard-wired to process. A cognitive concept requires an ability on the part of an individual to unify stimuli previously perceived as disparate. The kinds of stimuli most amenable to this kind of conceptualisation are visual and auditory stimuli. It is easy to see how different shapes can be grouped under a family concept (e.g. "triangular" or "symmetrical"). One can also classify sounds by their acoustic properties (e.g. "C" or "single note").
However, many invertebrates have a very weak capacity to discriminate between colours and sounds, and may therefore be unable to form most or all audiovisual concepts. The predominant sensory modality for these animals is usually smell. But how does one go about categorising smells? Although there are words in our language for individual smells, there are very few words that signify a common feature of disparate smells. ("Fragrant", "pungent" and "rank" are three possible examples.) The whole notion of what a family of related smells needs to be thought out carefully, before we can investigate the concept-forming abilities of animals (such as worms) whose sensory modality is predominantly olfactory rather than audiovisual.
"Aha!" Insight learning in honeybees
The sudden improvement in performance noted by Gould in categorical tasks (2002, p. 44) suggests a useful experimental way of identifying the presence of insight in animals. Cognitive tasks for animals should be designed with the aim of eliciting this kind of "Aha!" result.
Even more impressively, honeybees seem to be able to form highly abstract concepts such as "same" and "different", according to research by Giurfa, Zhang, Jenett, Menzel & Srinivasam (2001). The authors summarise their findings as follows:
The following report of the authors' research, suggesting that bees possess the concepts of sameness and difference, is taken from the San Francisco Chronicle (article by science writer K. Davidson, Thursday April 19, 2001):
Bees brighter than we knew, study finds. They pass cognitive tests usually given apes, people
Our pollen-hunting friends possess "higher cognitive functions," judging by cunning experiments in which the creatures learned to compare and distinguish different colors and patterns, according to today's issue of Nature.
In what an outside expert praises as "an exciting discovery," the French researcher Martin Giurfa and four colleagues showed that honeybees -- that's Apis mellifera to bee fanciers -- excel at cognitive tests normally performed by lab primates and human volunteers.
To demonstrate this, Giurfa and his team exposed bees to a simple Y-shaped maze. The entrance to the maze was marked with a particular symbol -- say, the color yellow.
As the Nature article shows, bees also can engage in abstract thought. The creatures can "master abstract inter-relationships," specifically the cognitive concepts of "sameness" and "difference," Giurfa and his team report. Hence, "higher cognitive functions are not a privilege of vertebrates," that is, creatures with backbones and much more complex nervous systems.
A bee flying through the entrance encountered a branching pathway. One branch was marked with the color yellow, another with the color blue. Bees that pursued the yellow-marked path discovered at its end a vial rich in sugar.
Bees that took the blue path got no sugar.
Normally, bees would have been just as likely to fly one way as another. But via Giurfa's experiment, the bees learned that sugar lay at the end of the route marked with the same symbol as that marking the outside entrance. In other words, "same" equals "sugar."
The bees demonstrated an ability to recognize "sameness" and "difference" - fundamental skills on any test of cognitive abilities.
In a second experiment, the bees showed they could apply the concepts of "sameness" and "difference" beyond what they had learned in the first experiment.
In subsequent experiments, the opening to the maze was marked by a different symbol -- such as vertical dark lines. In that case, on entering the maze the bees re-encountered the two pathways, which this time were marked not with colors but, rather, with lines -- vertical lines on one path, horizontal lines on the other.
Had the bees remembered the lesson of the first experiment, namely that "same" equals "sugar"? They had. In the second experiment, more than 70 percent of the bees promptly flew down the path marked by vertical dark lines, the same symbol as that above the entrance.
To perform the above task, it was necessary for the bees to acquire the rule that provides the goal of matching - "always choose the stimulus that is the same as the sample" - and it is also necessary to hold the information about the sample "in mind" to perform the test discrimination. Success in this task also presupposes sophisticated discrimination abilities in an insect and suggests the existence of an analogue of declarative memory (memory for facts). Honey bees have performed well in a range of DMS tasks, showing the ability to transfer their concept of "same" from one context (same colour) to another (same pattern of stripes) in successive trials, or vice versa.
One might attempt to account for the bees' DMS learning feats by positing that they are storing a "snapshot" of the sample in their memories, which elicits their response to a subsequent matching stimulus. On this account, the bees are not forming an abstract notion of "same", but simply matching pixels. However, the fact that bees can recognise and distinguish human letters, regardless of size, colour, position or font (Gould and Gould, 1988) refutes this "snapshot" hypothesis. Nor can such a hypothesis account for bees' abilities to identify general features of stimuli (e.g. asymmetry).
Even more impressively, honey bees are capable of solving a delayed non-matching to sample task, where they have to choose the stimulus that is different from the original sample (Giurfa, 2003).
The point that needs to be kept in mind here is that "sameness" and "difference" are not physical properties as such: they do not describe a measurable property of an object or group of objects. While one can describe what the bees are doing in a particular DMS task, using empirical terminology (e.g. the bees are looking for a stimulus whose colour matches the sample's), it is impossible to describe what the bees are doing in the ensemble of tasks, where they have to match colours or patterns, without employing abstract, non-empirical terminology (the bees are looking for a stimulus that is the same as the sample). The strategy for success in a DMS task has to be formulated at this level.
But why should a mind be needed to identify non-empirical properties? What I am proposing here is not that "non-empirical" equates to "mental", but that the identification of non-empirical properties is inherently mentalistic. Whereas empirical properties can be identified by some process of association and recall, non-empirical properties, such as sameness, have to be identified by looking for the rule which generates instantiations of these properties. The activity of attempting to follow a rule is a mentalistic one, as it can only be characterised in intentional terms.
Does the ability of honey bees to solve DMS tasks mean that they have the mental concepts of "same" and "different"? I suggest that bees have a concept of sameness, without knowing what they have concepts of, and without knowing that they have a concept of sameness. The last two abilities, but not the first, presuppose the possession of a language which is capable of expressing abstract concepts. There are no indications that bees possess such a language. (The phenomenon of bee "language" will be discussed in chapter 4.) The distinctions I have invoked here reflect Dretske's (1995) distinctions between being conscious of something (e.g. burning toast), being conscious of what you are conscious of, and being conscious that you are conscious of it.
Is a bee's concept of "sameness" the same as ours? I propose that this question can be resolved if we consider the following three questions: (i) do bees make the same responses as we do in DMS tests?, (ii) do they make responses for the same range of objects as we do?, and (iii) do they make appropriate responses for all objects that are empirically accessible to them? The answer to the first two questions is obviously negative: (i) bees cannot make the same responses, as their discriminatory abilities are different from ours (e.g. their vision is poorer than ours), and (ii) there are certain kinds of objects of which bees are unaware, as they can only be apprehended through abstract language. The third and more substantial question relates to whether they have a general concept of an "object", which they can apply to all kinds of stimuli that they can sense. Can they, for instance, apply the concept of "sameness" to smells, or only to visual stimuli? (If the latter is the case, then bees' concept of "sameness" is indeed a lower-level one than ours, as its scope is limited to one sensory mode.) And can they apply their concept across sensory modes - e.g. can they compare a visual stimulus (e.g. a disk marked with red paint) with a smell (e.g. a disk with no marks, impregnated with the smell of eucalyptus) and judge them to be different?
My proposals for testing the boundaries of bees' concepts of "sameness" and "difference"
The following suggestions relate to the question of whether bees can (i) generalise their concepts of sameness and difference across different senses and (ii) form a multi-modal concept of sameness. I propose the following two tests:
(ii) Can they form a multi-modal concept of sameness, such that if they enter a maze marked with a visual cue and an odour, and then encounter a fork with multiple branches, they fly down the one with the same visual cue and odour?Evidence for higher-order learning in insects
1. Meta-learning in insects: serial reversal learning
I'm not convinced that reversal learning is necessarily directly related to intelligence. It is possible that for some species, reversal is highly adaptive, and hence the good performance. In bees one could speculate that reversal is very important to an animal that forages on unstable resources. In bees and other social species this is even more critical since they use communal foraging strategies. Bees will follow other bees to resources previously identified. If these have dried out the new forager has to look for new resources fast or she will come back empty - a big waste of time (personal email communication, 19 August 2003).
L.18 The capacity for rapid reversal learning in an animal does not, by itself, warrant the ascription of mental states to it.
L.19 Progressive adjustments in serial reversal tests constitute good prima facie evidence that an animal is trying to adjust to sudden changes in its environment, by rapidly revising its expectations.
Drosophila can reversal learn and if the pattern-heat contingency is reversed, learning is faster (personal email communication, 11 August 2003).
2. Meta-learning in insects - concept formation
The learning curve is different from that of more standard tests in which bees are taught that a particular odor, color, or shape is always rewarded. During concept learning there is no evident improvement over chance performance until about the fifth or sixth test, whereas in normal learning there is incremental improvement beginning with the first test. This delay is characteristic of what has been called 'learning how to learn', which is interpreted as a kind of 'ah-ha' point at which the animal figures out the task.
L.20 An animal's ability to form categorical concepts and apply them to novel stimuli indicates the presence of mental processes - in particular, meta-learning.
...[R]ecent research has found that bees are capable of cognitive performances that were previously thought to occur only in some vertebrate species. For example, honeybees can interpolate visual information, exhibit associative recall, categorize visual information and learn contextual information. Here we show that honeybees can form "sameness" and "difference" concepts. They learn to solve delayed-matching-to-sample [DMS - V.T.] and delayed-non-matching to sample [DNMS - V.T.] discriminations and transfer the learned rules to novel stimuli of the same or a different sensory modality. Thus, bees can, not only learn specific objects and their physical parameters, but also master abstract interrelationships, such as "sameness" and "difference".
Bees are famously busy -- but they're also pretty brainy.
L.21 An animal's ability to identify non-empirical properties is a sufficient condition for its having mental states (intentional acts). Such an animal can apply non-emprical concepts, by following a rule.
(i) After being trained to enter a Y-shaped maze whose entrance is marked by a visual cue, and then fly down the arm of the maze marked with the same visual cue, can they generalise across modalities, and apply the "sameness" rule to a maze whose entrance is marked by a neutral odour, and which has branching arms, one of which has the same odour as the entrance?
Back to Chapter 2
Back to: Four models of a minimal mind in animals
*** SUMMARY of conclusions reached
References

