Liquids and Solids
Intermolecular Forces
Intermolecular Forces are relatively weak interactions that occur between molecules. There are three main types of forces:
Dipole-Dipole Forces
Molecules that have an asymmetric distribution of charge within the molecule are referred to as being polar. Since atoms differ in their ability to attract electrons to themselves (electronegativity), any situation in which two different atoms are bound together will result in an asymmetric distribution of charge. Almost every simple diatomic molecule consisting of two different kinds of atoms will be polar. The end of the molecule with the atom having the greater ability to attract electrons will be slightly negative and the other end will be slightly positive. The Dipole-dipole force then arises from the attraction between the slightly positive end of one molecule and the slightly negative end of another.
As a rule, dipole-dipole attractions are stronger than London forces although it's hard to make simple comparisons because polar molecules will exhibit both dipole-dipole and London forces.

Dipole-Dipole Forces animated.
Hydrogen Bonding

Illustrated above is an example of hydrogen bonding.
To fully understand hydrogen bonding, it's necessary to go back to the original idea of Coulombic forces. These are the attractive (or repulsive) forces between charged particles. The force of attraction between two opposite charges is proportional to the magnitude of their charges divided by the square of the distance between them. If we hook hydrogen up to an atom that is very good at attracting electrons (like N, O, or F) the hydrogen end of the bond becomes very positively charged and the other atom becomes negatively charged (i.e., polar). Now if we remember that hydrogen is the smallest atom on the periodic table, we realize that it is possible for two molecules to get very close together. The combination of high polarity and close approach result in the interaction being particularly strong. In fact, the interaction is so strong that it dwarfs all other dipole-dipole attractions and is given the special name of "hydrogen bonding".
London Dispersion Forces

London Dispersion Forces animated.
The simplest of the intermolecular forces, termed London dispersion forces after their discoverer Fritz London, arise (simplistically) from the weak attractive force of the electrons on one molecule for the nuclei of another molecule. There is no bond, i.e., no sharing of electrons between the two molecules.
Because London forces arise from interactions present between all molecules, these forces are always present. For non-polar molecules these are the only forces present. As a general rule for simple molecules, if a covalent bond is set at a relative value of 100, London forces have values between 0.001 and 0.1. In otherwords, very weak forces.
The Liquid State
When considering the three states of matter, the middle state is known as the Liquid State. It has some of the characteristics of the solid state and some of the characteristics of the gas state. Like the solid state, the liquid state tends to have very little ability to be compressed. Like the solid state, the liquid state is associated with somewhat low kinetic energy. Like the gas state, the liquid state tends to flow, or have fluidity. Like the gas state, the liquid state tends to take on the shape of the container that it is in. As the middle state, liquids are associated with the common phase changes freezing and boiling. In addition, other concepts such as viscosity, evaporation, and surface tension are also associated with the liquid state.
Structures and Types of Solids
There are 5 main
types of solids.
1. Ionic Solids
2. Covalent Solids
3. Polar Molecular Solids
4. Nonpolar Molecular Solids
5. Metallic Solids
To a large extent, the primary
difference between the types of solids is the mechanisms that hold them together
as solids. These mechanisms will be responsible for many physical
characteristics, such as melting points, boiling points, hardness and water
solubility.
Solid state crystal structures.

Carbon and silicon: Network Solids
Carbon exists as a pure element at room temperature in three different forms: graphite (the most stable form), diamond, and fullerene.
The most stable form of carbon is graphite. Graphite consists of sheets of carbon atoms covalently bonded together. These sheets are then stacked to form graphite.
Silicon Dioxide
Silicon dioxide (SiO2), also called silica, occurs naturally in many forms. Quartz is essentially pure silicon dioxide. Sand is composed of small quartz fragments. Many precious gems are quartz containing colored impurities. Amethyst is quartz colored red by the presence of iron(III) ions. Agate and onyx are also quartz containing impurities. Flint is silica colored black by carbon. Quartz has a very complicated crystal structure, which involves interwoven helical chains. All silicates involve silicon in tetrahedral environments surrounded by oxygen atoms.
Ionic Solids
Ionic solids are one common type of solids. In an ionic solid, the atoms are mostly in ionic form, and the ions are held together by the electrostatic attraction between the ions. Common properties of ionic substances are:
Vapor Pressure and Changes of State
A picture summarization of vapor pressure.
During vaporization, molecules in a liquid gain enough kinetic energy to break the bonds holding them to the rest of the molecules in the liquid and enter the gas. This can also occur in reverse: gas molecules can collide with the surface of the liquid and re-enter the liquid.
If you place a liquid in a closed container, at first there are very few molecules of the liquid in vapor form, and so the reverse process doesn't occur very often. As more of the liquid converts to vapor, the rate of the reverse process increases until finally there are the same number of molecules entering and leaving the liquid. Once this equilibrium is reached, the number of molecules in vapor form doesn't change, and we can compute the pressure of the vapor. The equilibrium is temperature dependent: as the temperature rises so does the vapor pressure. For example, at 25oC, water has a vapor pressure of 24 mmHg, at 100oC it has a vapor pressure of 760 mmHg.
In an open container, the vapor molecules can spread out to a great distance, and the liquid never reaches the equilibrium that it does in a closed container. This is why puddles left by a rainstorm evaporate over time.
Once you know the vapor pressure of a liquid at a certain temperature, you can use the ideal gas law to relate V and n to the pressure.
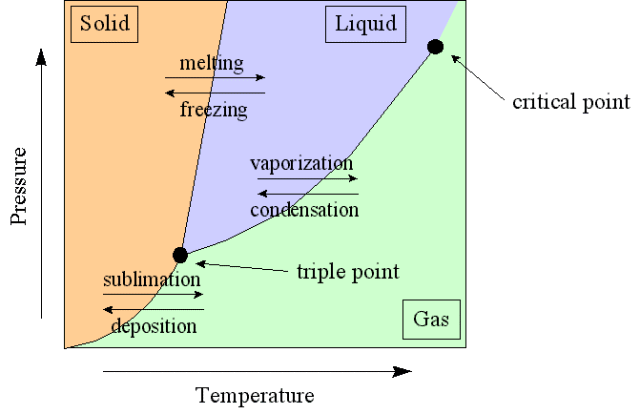
Phase Diagrams
Equilibrium can exist not only between the liquid and vapor phase of a substance but also between the solid and liquid phases, and the solid and gas phases of a substance.
A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance:
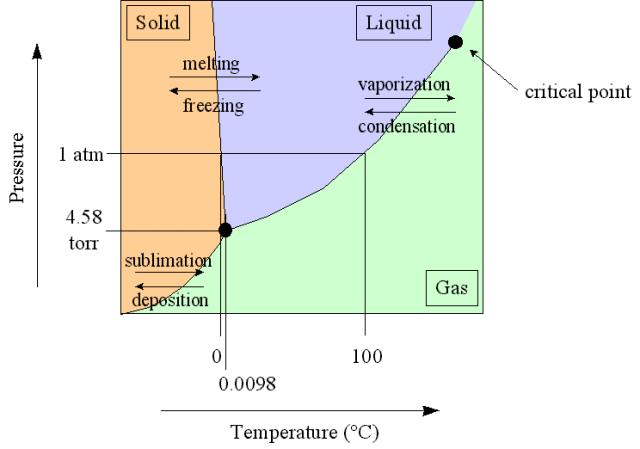
The curves indicate the conditions of temperature and pressure under which equilibrium between different phases of a substance can exist
The temperature above which the gas cannot be liquefied no matter how much pressure is applied (the kinetic energy simply is too great for attractive forces to overcome, regardless of the applied pressure)
Phase Diagram for Water
Increasing pressure will thus lower the temperature at which the solid will melt