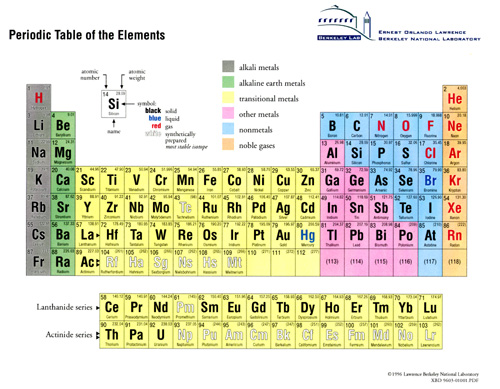
The history of the periodic table
The rectangular periodic table is familiar to anybody who has ever been in a science laboratory or classroom. This ingenious functional grouping of the chemical elements was created by several European scientists in the decade of the 1860's. In 1863, a 44 year old French geologist, A. E. Béguyer de Chancourtois created a list of the elements arranged by increasing atomic weight. The list was wrapped around a cylinder so that several sets of similar elements lined up, creating the first geometric representation of the periodic law.
In England, 32 year old analytical chemist John A. R. Newlands was also wrapping the elements, noting that chemical groups repeated every eight elements. He named this the octave rule, and compared it to a musical scale. Some less observant members of the English Chemical Society considered this absurd, so his work was ignored for years.
Chemists Dmitrii I. Mendeleev, a Russian, and German Lothar Meyer were working independently in 1868 and 1869 on the arrangement of elements into seven columns, corresponding to various chemical and physical properties. Their tables were similar - they acknowledged each other's work - the differences are subtle but important: Meyer's table was an accurate (for the time) accounting of the known facts about each element, such as melting point and atomic volume. The table clearly showed the existence of periodic chemical families. In 1870 Meyer's table and description of the periodic law was published in Liebig's Annalen.
A year earlier however, the 35 year old Mendeleev presented a much bolder and scientifically useful table. His paper, On the Relation of the Properties to the Atomic Weights of the Elements, was enthusiastically received by the Russian Chemical Society. In it, the periodic relationship between chemical groups, that is, elements with a similar stoichiometry of reaction, is clearly illustrated. In a scientific triumph, gaps in the table accurately predicted undiscovered elements.
Back
Periodic trends in atomic properties
