Relative Density and Specific Gravity
Relative density is a dimensionless ratio of the densities of two materials. The term specific gravity is similar, except that the reference material is water. A relative density can help quantify the buoyancy between two materials, or determine the density of one "unknown" material using the "known" density of another material. Mathematically, relative density is expressed as:
![]()
where G is
the relative density, and ρ is the densities of the two materials in the same
units (e.g., kg/m³, g/cm³).
Relative density is dimensionless, since it is a ratio between two quantities of
same unit. If the ratio is greater than 1, the object will be heavier than the
same volume of the reference. If it is less than 1, it will be lighter than the
reference. It is important to specify the reference material when reporting a
relative density, but when the reference material is not specified it is usually
understood to be water at 3.98 ° C.
Specific Gravity
Using water as a reference material can make the calculations using SI units convenient, since density of water is (approximately) 1000 kg/m³ or 1 g/cm³. Determining the specific gravity (same as relative density of an object relative to water) entails little effort, as the density of the object only needs to be divided by 1 or 1000, depending on the unit, e.g.:
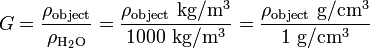
Difference between specific gravity and relative density
Specific gravity is a special case of relative density. While specific gravity has a reference density of water, relative density can have any reference density that is used. It is best to specify the reference material when using relative density, using subscripts:
Which states "the relative density of the object with respect to the reference material".
While relative density will not change as long as consistent units are maintained, the relative density is relative to its reference. The relative density of an object relative to mercury, is different than the relative density with respect to water (specific gravity).
Taking the relative density with respect to alcohol, the water has a specific gravity of 1.2, and the iron has a specific gravity of 10. Taking the relative density relative to water, the numbers would be .78, 1, and 7.9. With respect to iron, the numbers are .1, .12, and 1. These are all correct relative densities, however it is with different reference points. Note that again, units are not needed and hence these numbers are correct no matter what unit system is used.
Ours sponsor is House Design Software


