|
Electromagnetic Radiation |
Forms
of electromagnetic radiation
Infrared radiation
Beyond the red end of the visible
range but at frequencies higher than those of radar waves and microwaves is
the infrared region of the electromagnetic spectrum, between frequencies of
1012 and 5 1014 Hz (or wavelengths from 0.1 to 7.5 10-5 centimetre). William
Herschel, a German-born British musician and self-taught astronomer,
discovered this form of radiation in 1800 by exploring, with the aid of a
thermometer, sunlight dispersed into its colours by a glass prism. Infrared
radiation is absorbed and emitted by the rotations and vibrations of
chemically bonded atoms or groups of atoms and thus by many kinds of
materials. For instance, window glass that is transparent to visible light
absorbs infrared radiation by the vibration of its constituent atoms.
Infrared radiation is strongly absorbed by water, as shown in Figure 3.
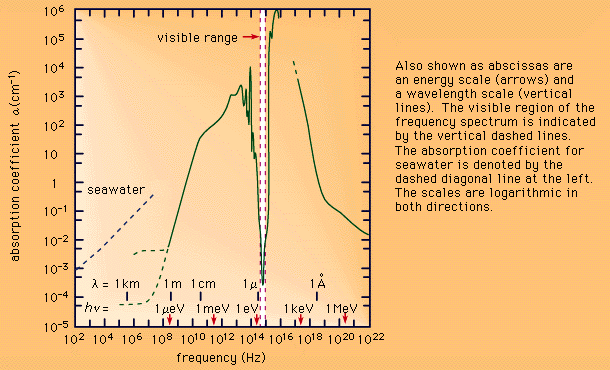
The absorption coefficient
for liquid water as a function of frequency.,and by the atmosphere. Although
invisible to the eye, infrared radiation can be detected as warmth by the
skin. Nearly 50 percent of the Sun's radiant energy is emitted in the
infrared region of the electromagnetic spectrum, with the rest primarily in
the visible region.
Atmospheric haze and certain
pollutants that scatter visible light are nearly transparent to parts of the
infrared spectrum because the scattering efficiency increases with the
fourth power of the frequency. Infrared photography of distant objects from
the air takes advantage of this phenomenon. For the same reason, infrared
astronomy enables researchers to observe cosmic objects through large clouds
of interstellar dust that scatter infrared radiation substantially less than
visible light. However, since water vapour, ozone, and carbon dioxide in the
atmosphere absorb large parts of the infrared spectrum most infrared
astronomical observations are carried out at high altitude by balloons,
rockets, or spacecraft.
An infrared photograph of a landscape
enhances objects according to their heat emission: blue sky and water appear
nearly black, whereas green foliage and unexposed skin show up brightly.
Infrared photography can reveal pathological tissue growths (thermography)
and defects in electronic systems and circuits due to their increased
emission of heat.
The infrared absorption and emission
characteristics of molecules and materials yield important information about
the size, shape, and chemical bonding of molecules and of atoms and ions in
solids. The energies of rotation and vibration are quantized in all systems.
The infrared radiation energy h emitted or absorbed by a given molecule or
substance is therefore a measure of the difference of some of the internal
energy states. These in turn are determined by the atomic weight and
molecular bonding forces. For this reason, infrared spectroscopy is a
powerful tool for determining the internal structure of molecules and
substances or, when such information is already known and tabulated, for
identifying the amounts of those species in a given sample. Infrared
spectroscopic techniques are often used to determine the composition and
hence the origin and age of archaeological specimens and for detecting
forgeries of art and other objects, which, when inspected under visible
light, resemble the originals.
Infrared radiation plays an important
role in heat transfer and is integral to the so-called greenhouse effect,
influencing the thermal radiation budget of the Earth on a global scale and
affecting nearly all biospheric activity. Virtually every object at the
Earth's surface emits electromagnetic radiation primarily in the infrared
region of the spectrum.
Man-made sources of infrared radiation include, besides hot objects,
infrared light-emitting diodes (LEDs) and lasers. LEDs are small,
inexpensive optoelectronic devices made of such semiconducting materials as
gallium arsenide. Infrared LEDs are employed as optoisolators and as light
sources in some fibre-optics-based communications systems (see
optoelectronic devices). Powerful optically pumped infrared lasers have been
developed using carbon dioxide and carbon monoxide. Carbon dioxide infrared
lasers are used to induce and alter chemical reactions and in isotope
separation. They also are employed in LIDAR (light radar) systems. Other
applications of infrared light include its use in the rangefinders of
automatic self-focusing cameras, security alarm systems, and night-vision
optical instruments.
Instruments for detecting infrared
radiation include heat-sensitive devices such as thermocouple detectors,
bolometers (some of these are cooled to temperatures close to absolute zero
so that the thermal radiation of the detector system itself is greatly
reduced), photovoltaic cells, and photoconductors. The latter are made of
semiconductor materials (e.g., silicon and lead sulfide) whose electrical
conductance increases when exposed to infrared radiation.
|