
The periodic table displays all of the chemical elements along with their chemical symbols and sometimes other information. The periodic table is arranged so that elements with similar properties fall into the same group, which forms columns in the periodic table. Group 1 would be located on the far left side of the periodic table, while Group 18, the noble gases, would be located on the far right side. The horizontal rows of the periodic tables are called periods.
The periodic table contains regions of similar elements, such as metals, which are Groups 1-12 and some from Groups 13-16, metalloids, nonmetals, which are most from Groups 14-17. and the noble gases, Group 18. Metals are elements that generally are solid at room temperature, have a grayish color and shiny surface, and conduct electricity. Metal is the largest region of the table, while nonmetals are the second largest region. Nonmetals are chemical elements that are neither a metal, metalloid, or a noble gas. Metalloids are elements having properties of metals as well as nonmetals. Noble gases are elements that exist in the gaseous state at normal temperature and are nonreactive with other elements. Noble gases are located on Group 18 in the periodic table.
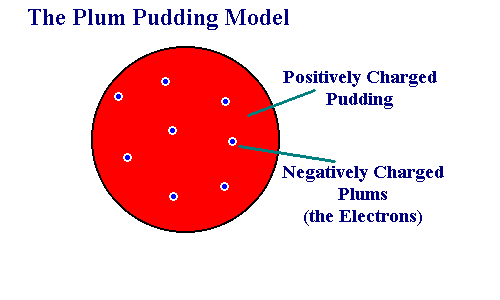
Elements are made up of three important particles, which are the protons, neutrons, and electrons. The atom is not indivisible but is a small particle. In 1897, the English physicist JJ Thompson made an atomic model, which was called the "plum pudding model," that faetured negatively charged electrons embedded in a ball of positive charge. Niels Bohr's model shows that the energy of an electron in an atom is quantized, meaning it cannot have any arbitrary value of energy.
Electrons are recognized as particles or waves. When electrons are at their lowest possible energy level, an atom is said to be in a ground state, the lowest energy state of a quantized system. When the electrons absorbs energy to get into the next highest energy level, an atom is said to be in an excited state, the condition of an atom in a state higher than the ground state.
The visible spectrum is a small part of the electromagnetic spectrum, the total range of electromagnetic radiation ranging from the longest radio waves to the shortest gamma rays. Electromagnetic spectrum consists of all types of waves, includign microwaves, radio waves, X rays, gamma rays, infrared radiation, and ultraviolet radiation, and all are electromagnetic vibrations traveling through space in the form of waves. However, all waves have different wavelength and frequency.
The quantum theory, the field of physics based on the idea that energy is quantized and that this has significant effects on the atomic level, uses the complex mathematical equations of quantum mechanics that describe waves. The quantum mechanical model can predict the quantized energy levels for electrons, but the model of today does not tell the exact path electrons take around the nucleus. Today's quantum mechanical model scientists use concerns finding the probability of certain positions of electrons. An orbital, a region of an atom in which there is a high probability of finding electrons, can show positions where up to two electrons can be found.
The periodic table is organized by the atomic numbers of elements. The number of protons an element has is its atomic number. Hydrogen would have 1 proton since it's atomic number is 1, Helium, atomic number 2, would have 2 protons, and so on. Unlike the number of protons, the number of neutrons in an element varies. Atoms with the same number of protons but different numbers of neutrons are called isotopes. The total number of protons and neutrons in an atom is called the mass number. With the mass number, finding the number of neutrons in an atom is easy, since the number of protons in different atoms of the same element stay fixed.
An electron configuration is an abbreviated form of an orbital diagram of an atom. The electron configuration of Hydrogen would be 1s1, the coefficient 1 meaning that the electron is located in the first energy level and the superscript 1 representing one electron occupying the s orbital in that energy level. Electrons are arranged by Hund's rule, which states that orbitals of equal energy are each occupied by one electron before any pairing occurs by adding a second electron. Repulsions between electrons in one orbital remain minimized in accordance to Hund's rule. Electron configuration can also be shorten in terms of noble gases. For example, the whole electron configuration of Sodium, Na, is 1s22s22p63s1. But Sodium's electron configuration can be shorten as [Ne]3s1, Neon (Ne) being the last noble gas in the periodic table before Sodium.
Main menu
Topic 1: Matter and Energy
Topic 3: Periodicity
Credits and Links