Topic 11: Electrochemistry
How does electric current flow?
When electrons transfer, energy is released and electricity is produced. While an atom and an ion are suspended onto each other, when electrons are exchanged between two atoms, heat energy is released, causing the temperature to increase. As the redox reaction slows, the temperature goes low. But if the reactants are separated, electric energy is released instead. The porous barriers, a medium through which ions can slowly pass, prevents the two reactants from mixing with each other but electrons can still transfer as electricity. This can take place in voltaic cells, electrochemical cells that produce electricity. In the wire of the external circuit, the negative electrons go from anode to cathode. Voltaic cells are made from two separate components, half-cells, labeled anode and cathode. Oxidation or reduction can occur in half-cells. An oxidations is a loss of electrons by an atom or an algebraic increase in its oxidation state, and reduction is the gain of electrons. Both must occur together, because the number of electrons lost must equal the number gained. This makes a redox reaction.
How are electron transfers described?
The oxidation state, or an oxidation number, represents an atom's share of the bonding eletrons. Ionic compounds have oxidation numbers equal to the ionic charge, while the oxidation number of covalent compounds is the average charge for an atom according to electronegativities. Knowing oxidation numbers are useful for identifying half-reactions and balancing redox equations.
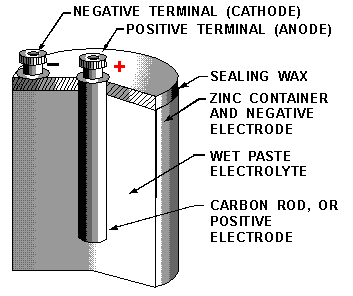
How do batteries work?
A battery is either a single voltaic cell or a group of voltaic cells connected together. Batteries depend on redox reactions where chemical energy converts into electric energy. A dry cell battery, a zinc-carbon cell, is filled with moist MnO2 paste and NH4Cl paste. Alkaline batteries are different from dry cell batteries because they are bridged by KOH paste.
Main menu
Topic 10: Acids and Bases
Topic 12: Nuclear Chemistry
Credits and Links
