| Preliminary Outline |
| Alkylating
Agents |
The adduct-forming agents include the alkylating agents and platinum compounds.The alkylating agents damage DNA by adding alkyl adducts to DNA bases, at either one or two sites per drug molecule. The platinum compounds form similar adducts.
Monofunctional Alkylating Agents
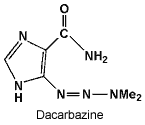 The
monofunctional alkylating agents alkylate at a single DNA site, and include
dacarbazine, streptozotocin and procarbazine. They are used to treat Hodgkin's
and other lymphomas.
The
monofunctional alkylating agents alkylate at a single DNA site, and include
dacarbazine, streptozotocin and procarbazine. They are used to treat Hodgkin's
and other lymphomas.
Dacarbazine requires activation in the liver, although the active metabolite is unknown. It inhibits DNA, RNA and protein synthesis, probably by methylation of DNA at N7 or O6 of guanine.
Streptozocine does not require metabolic activation, and 7% of its methylation of DNA occur at guanine O6. It is used primarily to treat pancreatic islet cell carcinoma, and will induce diabetes in experimental rats.
Procarbazine requires activation by cytochrome P450, and the postulated final active metabolite is a diazomethane. Its most common use is in Hogdkin's lymphoma, in the MOPP therapy.
Nitrogen Mustrads
The general mechanism of action of the nitrogen mustards is the formation of ethylene ammonium ions capable of forming a covalent bond with macromolecules. These ions form crosslinks between complementary strands of DNA and between DNA and protein, and induce alkylation of proteins.
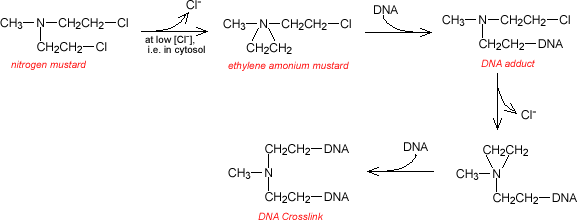
The chloride ion is not
displaced in plasma because the chloride concentration is relatively high. But
once inside the cell, the chloride concentration is low and the Cl![]() dissociates forming the ethylene ammonium. Alkylated bases may undergo a ring
opening step which is subsequently repaired.
dissociates forming the ethylene ammonium. Alkylated bases may undergo a ring
opening step which is subsequently repaired.
The nitrogen mustards include nitrogen mustard, chlorambucil, cyclophosphamide and melphalam. Nitrogen mustard acts mostly by forming DNA inter- and intrastrand crosslinks and DNA-protein crosslinks. The primary site of alkylation is the guanine N7 position, and the N7 position of an adjacent guanine or adenine. In vitro, peak DNA damage by nitrogen mustard occurs within the first hour, and lesions are repaired in 12-24 hours.
The primary use of nitrogen mustard is the treatment of Hodgkin's lymphoma in the MOPP regime. It is very reactive in aqueous solution and free drug cannot be detected in plasma after a few minutes of IV infusion. The main side effects are menstrual irregularities in females and sterility in males.
Chlorambucil is the drug of choice for the treatment of chronic lymphocytic leukemia, as well as lymphomas. A cyclohexyl ring provides increased stability that allows oral administration. The plasma half life is 1 hour.
Cyclophosphamide is one of the most stable nitrogen mustards, and requires cytochrome P450 activation to 4-hydroxycyclophosphamide. It's half-life is about 7 hours and its crosslinking effect may peak 6-12 hours after exposure. Cyclophosphamide can be metabolized to acrolein, which is toxic to the bladder.
Melphalan's structure is composed of nitrogen mustard linked to the amino acid phenylalanine. It was designed to be actively transported into th cell via the phenylalanine transporter, but it is taken up by the leucine transporter instead. Plasma half-life after IV administration is 90 minutes. Surprisingly, nausea and vomiting are infrequent with melphalan. It is very active against breast and ovarian cancers as well as myeloma.
 Other
Alkylating Agents
Other
Alkylating Agents
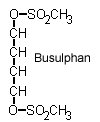
Other alkylating agents include busulfan, the chlororethylnitrosoureas and AZQ.
Busulfan is one of a series of alkyl sulfonates where the internal alkyl chain is 2-9 carbons long. It forms DNA crosslinks and is used to treat chronic granulocytic leukemia.
The chloroethylnitrosoureas include BCNU, CCNU, MeCCNU and the experimental agent chlorozotocin. They decompose under physiological conditions to produce alkylating moieties, which can further decompose to chloroethylcarbonium ions that produce DNA inter and intrastrand crosslinks and DNA-protein crosslinks.
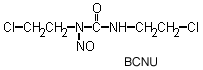 The
chloroethylnitrosoureas are highly lipophylic and readily cross the blood-brain
barrier, thus are the most widely used agents to treat brain tumors and leukemia,
especially BCNU. A breakdown product, isocyanate, can inactivate some enzymes
and is the major source of toxicity, especially renal in children.
The
chloroethylnitrosoureas are highly lipophylic and readily cross the blood-brain
barrier, thus are the most widely used agents to treat brain tumors and leukemia,
especially BCNU. A breakdown product, isocyanate, can inactivate some enzymes
and is the major source of toxicity, especially renal in children.
 Aziridylbenzoquinone,
AZQ, was designed to be highly lipophilic in order to treat primary brain tumors.
It is also reduces the number of blast cells in the cerebrospinal fluid of childhood
leukemia patients. AZQ is though to be more active in hypoxic tumors because
it is reduced under hypoxic conditions. The reduced AZQ produces strand breaks
and DNA crosslinks.
Aziridylbenzoquinone,
AZQ, was designed to be highly lipophilic in order to treat primary brain tumors.
It is also reduces the number of blast cells in the cerebrospinal fluid of childhood
leukemia patients. AZQ is though to be more active in hypoxic tumors because
it is reduced under hypoxic conditions. The reduced AZQ produces strand breaks
and DNA crosslinks.
Platinum Compounds
Cisplatin and carboplatin bind to DNA with high G-C content, and are thought to affect its template function. They act by a mechanism similar to alkylating agents, except they form platinum adducts instead of alkyl adducts.
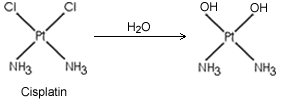 Cisplating
and carboplatin need to be hydroxylated by reacting with water before they can
form adducts. Carboplatin reacts with water much slower than cisplating, thus
its peak action is delayed about 12 hours compared with cisplatin. Both compounds
can cur testicular cancer, and are also effective against cancer of the ovary,
head and neck, and small cell lung cancer.
Cisplating
and carboplatin need to be hydroxylated by reacting with water before they can
form adducts. Carboplatin reacts with water much slower than cisplating, thus
its peak action is delayed about 12 hours compared with cisplatin. Both compounds
can cur testicular cancer, and are also effective against cancer of the ovary,
head and neck, and small cell lung cancer.
Binding to sulfur-containing molecules can prevent the reaction of platinum compounds with DNA. High intracellular concentrations of glutathione may confer resistance.
Both cisplatin and carboplatin are excreted via the kidneys, and are nephrotoxic. Hypertonic saline (NaCl is administered to pretect the kidneys. Cisplatin is not myelosuppresive, but may produce hearing problems or peripheral neuropathy.
![]()
![]() Continue
to "Antimetabolite Agents" or take a
quiz: [Q1].
Continue
to "Antimetabolite Agents" or take a
quiz: [Q1].
Need more practice? Answer the review questions below.
Questions coming soon