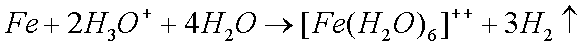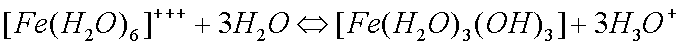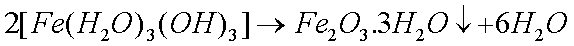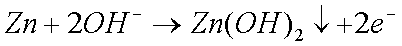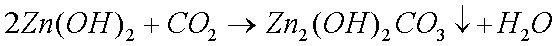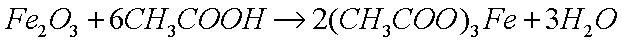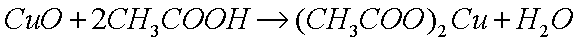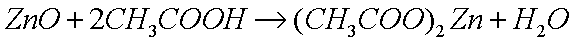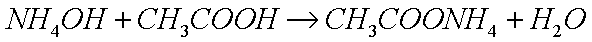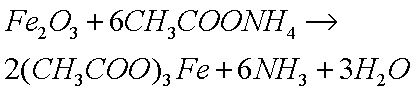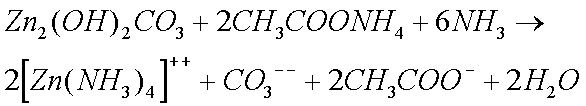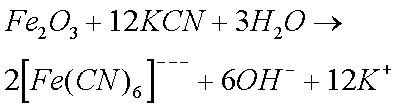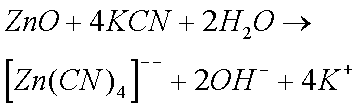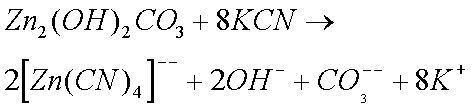Some copper will also oxidize in the presence of moisture to form greencopper sulfate when oxygen and sulfur dioxide from the air aredissolved in the water:
 |
and other similar compounds. You are likely to see this on clocks
that perform their service on ships at sea and also that perform
their service outdoors and near the sea, where there is plenty of
salty moisture in the air to act as the electrolyte.
Brass in clocks that perform their service indoors and in areas of
low moisture develop a layer of copper (II) and zinc (II) oxides,
which we see as the brown discolouring. The layer of oxides
protects the metal underneath. However, if the clock were not
maintained, the lubricants fail and the pivots are allowed to grind
away at the bushing surfaces, you would find a deposit of black
powder on the base (usually the piece of wood that the mechanism
is mounted on). This black powder is primarily copper (II) oxide
and some zinc (II) oxide.
Cleaning:
Since the oxide layer protects the brass underneath, it is better to
clean the clock without disturbing the oxide layer. Unfortunately,
people think that clean brass must have that shiny yellow colour, so
clock repairers polish the brass and remove the oxide layer to make
the brass look beautiful and impress the customer. The best way to
clean brass is to use either detergent cleaners without ammonia and
without acids or to use organic solvents, such as acetone or
tetrachloroethylene, to remove the dirt and oil residues (but not the
oxide layer!) The only situation, in which it would make sense to
remove the oxide layer, would be if you intended to apply a
protective layer of paint or lacquer to the surface of the brass.
Polishing:
The method for polishing metals like copper, brass, and steel, is by
removing the layer of oxide, which results in a small loss of metal
atoms as the oxides are dissolved. The most common method is to
dissolve the oxide in a weak acid, such as acetic acid (glacial
vinegar), which reacts with the basic (alkaline) oxide to produce
the metal acetate (a salt) and water:
The weak acid will react with the oxide but will not react
significantly with the unoxidized metals in the few minutes it
would take to polish the metals. If left for an extended period of
time in a solution of acetic acid, the metals would react. After the
oxide layer is removed, the metal is rinsed in ammonia solution to
remove any remaining acid and then rinsed in water (and dried
immediately!)
Ammonia:
Since acids are oxidizing agents and bases (alkalis) are reducing
agents, it would make a lot more sense if the oxide (alkaline) layer
could be removed with a base (or in an alkaline medium).
However, oxides do not react with bases. If you have a weak base,
such as ammonium hydroxide (acqueous ammonia), and you add
acid (such that there is much more base than there is acid, resulting
in a net alkaline solution), such as acetic acid, you get the
ammonium salt of that acid, namely ammonium acetate:
which reacts with metal oxides to form the metallic salts of that acid:
Zinc and Copper ions form complex ions with ammonia solutions.
Note, however, that the ammonium ion is not as strongly acidic as the hydrogen ion it replaces, which is why it does not remove iron oxides as readily as the hydrogen ion (in acetic acid) at room temperature. This is one of several problems I have experienced while cleaning clocks.
The Zinc Carbonate and Copper Sulphate, that are formed when clocks are in humid conditions, also form complex ions with ammonia solutions.
Cyanide:
Sodium and Potassium Cyanides are particularly effective in removing rust and Zinc Oxide (and Carbonate). Cyanides were therefore widely used by horologists until their use was banned (they are extremely toxic). The reason why cyanides are so effective is because of the formation of complex ions. (I do not believe that cyanides form complex ions with copper ions.)
Go to Cleaning and Polishing Brass (III)
Clock Repair Main Page
Escapements in Motion
Links Page