There is a pH differential between arterial blood (pH=7.4) and intracellular fluid (pH = 7.0). Most metabolic reactions liberate H+, and a buffer system is needed to maintain physiological pH. The main physiological buffers are:
The phosphate buffer is very effective but not found in high concentrations in all tissues. The bicarbonate buffer is also very effective and there are high levels of bicarbonate in all tissues that contain carbonic anhydrase (red blood cells, kidney, pancreas, stomach and brain):
carbonic
anhydrase
CO2 ![]() H2CO3
H2CO3 ![]() HCO3(-) + H+
HCO3(-) + H+
The respiratory buffering system uses bicarbonate. The respiratory system controls CO2 levels, while the kidney can excrete bicarbonate. Hyperventilation leads to loss of CO2 and creates alkaline conditions, while hypoventilation creates acid conditions. Peripheral receptors detect CO2 concentration changes and send the appropriate signal to the respiratory system. When CO2 builds up, a central receptor increases ventilation even if pH receptor is sensing high pH.
Combinations of these and other minor buffers in different compartments make up three different buffering systems: the blood buffering system, the respiratory buffering system and renal buffering system.
The blood buffering system uses three different chemical buffers: phosphate, bicarbonate and proteins. The phosphate buffer is not abundant in blood. Blood contains a high concentration of proteins.
R—CH—COOH
![]() R—CH—COO-
+ H+
R—CH—COO-
+ H+
|
|
NH2
NH2
R—CH—COOH
+ H+ ![]() R—CH—COOH
R—CH—COOH
|
|
NH2
NH3(+)
 Histidine |
In addition to the acid/base properties of their N-terminus and C-terminus, their side chains are very effective buffers in a large range of pH because of their different pKa. Histidine side chains are specially good at picking up H+. It is abundant in hemoglobin.
The renal buffer system uses bicarbonate, ammonium, phosphate and other titrable acids (phosphate and other minor buffers are called titrable acids). In the kidneys, the bicarbonate buffer may increase systemic pH in three ways: secrete H+, "reabsorb" bicarbonate, or produce bicarbonate. H+ secretion occurs mostly in the proximal tubule by the carbonic anhydrase reaction. Proximal tubule cells have this enzyme both in the cytosol and in the lumen side of the apical membrane. In acidic conditions, CO2 diffuses inside tubular cells and is converted to carbonic acid, which the dissociates to yield a proton. The proton is secreted into the lumen by the Na+/H+ antiporter.
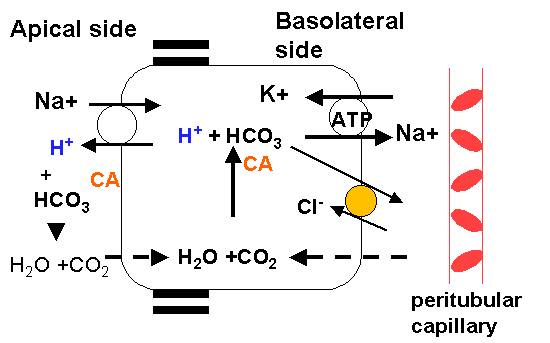 There
are no bicarbonate trransporters in the apical membrane of tubular cells, so
it cannot be directly reabsorbed. There are transporters in the basolateral
membrane, so once bicarbonate is made in the cell it can be reabsorbed. If there
is bicarbonate in the filtrate, the secreted H+ will combine with it to form
carbonic acid by catalysis of apical membrane carbonic anhydrase. The CO2 will
then enter the cell by diffusion and be transformed again into bicarbonate by
cytosolic carbonic anhydrase. Bicarbonate can then be transported across the
basolateral membrane.
There
are no bicarbonate trransporters in the apical membrane of tubular cells, so
it cannot be directly reabsorbed. There are transporters in the basolateral
membrane, so once bicarbonate is made in the cell it can be reabsorbed. If there
is bicarbonate in the filtrate, the secreted H+ will combine with it to form
carbonic acid by catalysis of apical membrane carbonic anhydrase. The CO2 will
then enter the cell by diffusion and be transformed again into bicarbonate by
cytosolic carbonic anhydrase. Bicarbonate can then be transported across the
basolateral membrane.
 The
kidneys also make bicarbonate at the collecting duct. This reaction is driven
by the diffusion of CO2 into the cell (or produced by the cell's own metabolism)
and an ATPase that pumps H+ into the filtrate.
The
kidneys also make bicarbonate at the collecting duct. This reaction is driven
by the diffusion of CO2 into the cell (or produced by the cell's own metabolism)
and an ATPase that pumps H+ into the filtrate.
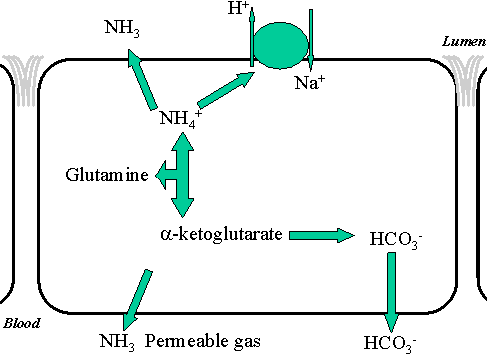
The phosphate buffer in the kidney can pick up H+ and form phosphate to be excreted in the urine. Alternatively, the H+ can be picked up by the ammonia system in a complicated set of reactions. The kidney makes ammonia by breaking down glutamine. Ammonium is secreted into the filtrate while the other products are reabsorbed.
 Disturbances
of the acid/base balance will lead to respiratory or metabolic alkalosis or
acidosis. Respiratory acidosis is due to an accumulation of CO2 in the
blood stream. This pushed the carbonic anhydrase reaction to the right, generating
H+:
Disturbances
of the acid/base balance will lead to respiratory or metabolic alkalosis or
acidosis. Respiratory acidosis is due to an accumulation of CO2 in the
blood stream. This pushed the carbonic anhydrase reaction to the right, generating
H+:
ca
CO2 ![]() H2CO3
H2CO3 ![]() HCO3(-) + H+
HCO3(-) + H+
This may happen when there is something wrong with the lungs: an accident were the breathing muscles are damage (decreased ventilation), barbiturate overdose, airway obstruction or lung disease (pneumonia, cystic fibrosis, emphysema, etc.). Even if the peripheral receptors sense the change in pH, the lungs are unresponsive. The kidneys will compensate by secreting H+. If H+ excretion cannot restore the balance, the kidneys will also generate bicarbonate. The most common clinical intervention is IV bicarbonate.
Respiratory alkalosis is generally caused by hyperventilation, usually due to anxiety. Fever or aspirin toxicity may also cause respiratory alkalosis. The body will reduce the breathing rate, and the kidney will excrete bicarbonate. If there is no H+ excreted in the kidneys, therefore cannot reabsorb of bicarbonate and will be excreted. The easiest way to treat respiratory alkalosis is to breath into a paper bag, i.e. recirculating CO2.
Metabolic acidosis is the
gain of a fixed acid (i.e. anything but carbonic acid), or the loss of bicarbonate.
Usual causes are the generation of ketone bodies in uncontrolled diabetes or
diarrhea (intestinal content is alkaline), or excess alcohol consumption (alcohol
![]() formaldehyde
formaldehyde ![]() acetic acid) . Exercise creates a milder, transient metabolic acidosis because
of the production of lactic acid. The body will compensate with hyperventilation
and increased bicarbonate reabsorption in the kidney. Clinical treatment is
IV bicarbonate.
acetic acid) . Exercise creates a milder, transient metabolic acidosis because
of the production of lactic acid. The body will compensate with hyperventilation
and increased bicarbonate reabsorption in the kidney. Clinical treatment is
IV bicarbonate.
Metabolic alkalosis is due to the gain of base other than bicarbonate or the loss of acid other than carbonic. The most common causes are excessive ingestion of sodium bicarbonate as an antiacid, or excessive vomiting. The respiratory system will hypoventilate but this will not be effective because CO2 will accumulate and the CO2 receptors will override the pH receptors. The kidney will make more of a difference by not reabsorbing bicarbonate.
Celebrate, this is the last lecture! or take a quiz: [Q1] [Q2].
Need more practice? Answer the review questions below.
1-