Unlike all of the other types of stereochemistry discussed here, stereochemistry associated with a double bond is invariant with regard to a mirror plane. Instead, double-bond stereochemistry is directly related to the positioning of substituents relative to the double bond itself. Double bonds (and related systems) have a maximum of four substituents, a maximum of two on each end of the double bond. Compared to a substituent on the other end of the double bond, a substituent on one end may be on the same (cis) or the opposite (trans) side. This description implies that rotation around the double bond is hindered, and that the positions of the two substituents on one end are indeed fixed relative to the other end. Determination of whether a given double bond really does have hindered rotation is beyond the scope of this document; if the double bond does not have hindered rotation, it does not have double-bond stereochemistry and the depiction recommendations discussed here would not apply.
Double bonds are planar, and so should preferably be depicted with plain bonds at all four substituents.
| PREFERRED | ACCEPTABLE |
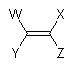 |
|
Substituents consisting of a hydrogen atom may be omitted according to standard conventions regarding the use of implicit hydrogens. Substituents consisting of a lone pair will also generally be omitted. When a substituent is omitted from the drawing, it is assumed to be positioned on the side of the double bond opposite to an existing substituent on the same atom.
| PREFERRED | ACCEPTABLE |
|
|
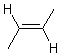 |
|
|
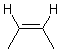 |
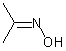 |
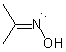 |
For sterically unstrained, acyclic double bonds, the angle between substitutents should generally be close to 120° as shown. Double bonds with highly congested substituents and double bonds in rings other than six-membered rings will commonly have angles other than 120°. In no case, however, should two substituents on the same atom of a double bond be positioned on the same side of the bond.
| NOT ACCEPTABLE |
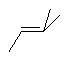 |
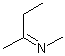
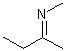
Asymmetric double bonds should never be drawn with implicit substituents, that is, where one or more substituents are "contracted" into an atom label at one end of the bond or the other. The substituents should always be drawn explicitly. This is particularly true when the double bond is oriented vertically, since the horizontal nature of atom labels may imply a cis/trans relationship where one was not intended.
| PREFERRED | PREFERRED | PREFERRED | NOT ACCEPTABLE | NOT ACCEPTABLE |
 |
 |
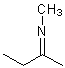 |
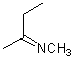 |
|
Double-bond-like systems are not restricted to isolated double bonds. The same issues apply to any systems containing four coplanar substituents with two on each end of a central axis. Extended cumulenic systems with an odd number of bonds describe one such family (similar systems with an even number of double bonds have tetrahedral stereochemistry instead).
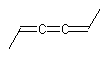
(E)-hexa-2,3,4-triene
In some cases, particularly in perspective drawings and in other bridged ring systems, it may be necessary to draw a double bond viewed from a non-standard orientation. Whenever possible in such cases, the local configuration of the double bond as drawn should match the actual configuration intended. That is, a cis double bond with two substituents should always be drawn with both of those substituents on the same side of the bond, regardless of the orientation of the bond itself.
| PREFERRED | NOT ACCEPTABLE |
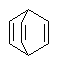 |
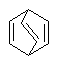 |
That said, a viewer should generally expect that any double bond within a ring containing six or fewer atoms will be in a cis configuration relative to the ring. In small rings of this type, the depiction of a trans bond when it could otherwise be drawn as cis only adds extra confusion for the viewer.
Interpretation is much more of an issue in slightly larger ring systems where both cis and trans orientations are possible. Although small ring systems are generally drawn as convex polygons, larger rings containing 9 or more atoms are preferentially drawn as non-convex polygons, with four or more bonds oriented trans rather than cis. Double bonds in large rings must always be drawn in the configuration that is intended, even if that requires that the ring system itself be depicted in a different shape.
| PREFERRED | NOT ACCEPTABLE |
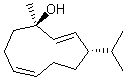 |
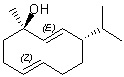 |
It is often useful to be able to depict a double bond of unspecified configuration, such as the structure of "but-2-ene" as distinct from "cis-but-2-ene" and "trans-but-2-ene". There are at least three different conventions that have been used to depict these configurations.
The simplest option is to draw the double bond and its substituents linearly.
This depiction style should only be used when the double bond has exactly two
substituents, one on each end. When possible, the double bond itself should be
arranged horizontally. If the entire structure is linear, it may be represented
in textual form instead, without any explicit bonds. In practice, linear styles
are primarily used for small compound, although they are acceptable for
compounds of any size.
| PREFERRED | PREFERRED | NOT ACCEPTABLE | NOT ACCEPTABLE |
|
|
|
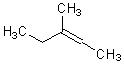 |
| ACCEPTABLE | NOT ACCEPTABLE | NOT ACCEPTABLE |
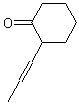 |
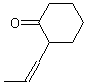 |
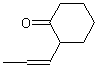 |
The most common convention for depicting unspecified double bonds in compounds of all sizes uses a wavy bond adjacent to the double bond. Under no circumstances, however, should the wavy bond join the double bond to another stereogenic center.
| PREFERRED | NOT ACCEPTABLE |
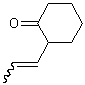 |
 |
This style is usually used only in cases where the double bond has a single substituent one or both ends; the wavy bond would preferably be positioned on that substituent. .
| PREFERRED | ACCEPTABLE |
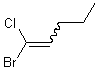 |
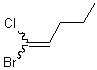 |
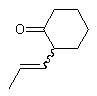
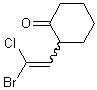
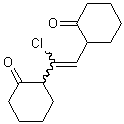
If that single substituent connects the bond to another stereogenic center, however, the wavy bond must not be positioned there, and instead should be placed on the other end of the double bond.
| PREFERRED | NOT ACCEPTABLE |
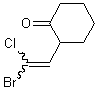 |
 |
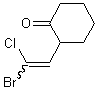
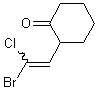
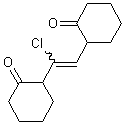
If a wavy bond is used on the end of a double bond that has two substituents, both substituents should preferably be depicted with a wavy bond unless one of the substituents connects the double bond to another stererogenic center.
| PREFERRED | ACCEPTABLE | ACCEPTABLE |
 |
 |
 |
| PREFERRED | NOT ACCEPTABLE |
 |
 |
In rare cases, it is impossible to depict an unspecified double bond either by drawing the substituents linearly or by using wavy bonds. In those cases, it is clearest to depict the two configurations as separate structures separated by the word "or".
| PREFERRED |
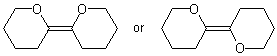 |
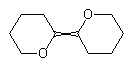
A crossed double bond has in the past been used to indicate unspecified double bond configurations. This type of bond is no longer considered acceptable for general use, although it may still be required by some computer software.
| NOT ACCEPTABLE | NOT ACCEPTABLE |
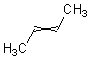 |
 |