
 |
|||||||
| Solid | Liquid | Gas | Synthetic | ||||
|
|
Alkali Metals |
|
Alkali Earth Metals |
|
Transition Metals |
|
Rare Earth Metals |
|
|
Other Metals |
|
Noble Gases |
|
Halogens |
|
Other Non-Metals |
|
Brief History
|
|
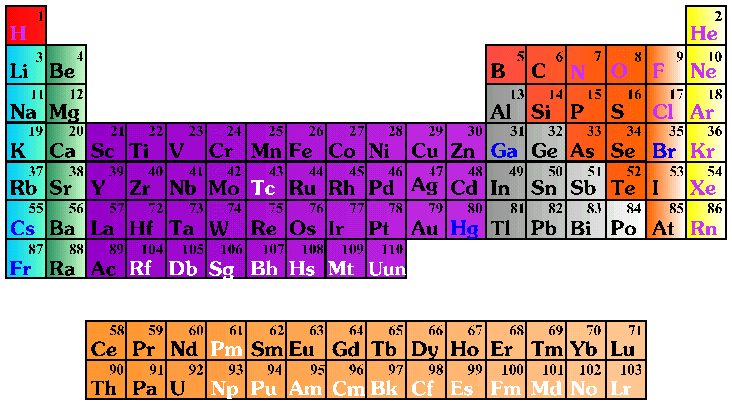
The periodic tables we see on the laboratory walls today, owe their existance to a flurry of activity in Europe in the 1860s. Several scientists were working on various models listing the elements and their atomic weight, and the known facts at that time. An English analytical chemist called John A. R. Newlands produced a chart wrapped around a cylinder. A French geologist A. E. Beguyer de Chancourtois produced a similar chart with the elements listed in increasing atomic weight. A Russian and a German chemist both went the rectangular chart route, with seven columns of elements arranged in various chemical & physical properties. German Lothar Meyer produced a very accurate chart listing the melting point, atomic volume and other known facts. He published his table in 1870 in Leibig's Annalen. However Russian chemist Dmitrii I. Mendeleev had published his paper "On the Relation of the Properties to the Atomic Weights of the Elements" a year ealier with his chart clearly illustrating the periodic relationship between chemical groups, and with great forsight had even left gaps for the elements yet to be discovered. 133 years later, the modern tables clearly show the influence of Mendeleev's model. |
 |
| "What's the most important thing to learn in chemistry? Never lick the spoon" |
![]()
|
Office
|
Quiz
|
![]()