
Objective: To evaluate clinical factors that can influence the results of spirometry in cured tuberculosis patients.Predictive Factors of Poor Lung Function in Cured Tuberculosis Patients Mohamed Saleh Al-Hajjaj, MD, FRCP(C)*
Design: Measurement of spirometry in treated tuberculosis patients with review of patient’s records for duration of symptoms, chest radiography and residual changes within five years of completion of anti-tuberculosis therapy.
Setting: Sahary Chest Hospital, Riyadh, Saudi Arabia
Results: Forty-six patients were studied (23 males, 23 females). Forced Vital Capacity (FVC), Forced Expiratory Volume at end /1 Sec (FEV1) and ratio of FEV1/FVC were abnormal in 13 cases, and oxygen saturation was less than 90% in three cases. Significantly higher rate of abnormal spirometry was observed in patients with poor compliance (poor 58.3% Vs good 17.6%), patients with duration of treatment less than 6 months (<6m 53.3% Vs ?6m 16.1%), and patients with advanced lung damage (advanced 38.5% Vs mild 10%).
Conclusion: Poor compliance with the prescribed drug regimen, and significant lung damage seen on radiography were found to adversely affect the degree of loss of lung function. Furthermore, lung functions appear to improve with time, and the longer the duration of recovery from tuberculosis.
Bahrain Med Bull 2002;24(1):19-22.
Pulmonary tuberculosis is endemic in Saudi Arabia with estimated annual prevalence of 30 cases per 100,000 population1. Despite major improvement in health care and particularly in the treatment of tuberculosis, sequelae of the pulmonary disease continue to be frequently encountered in clinical practice2. Post tuberculous lung diseases including bronchiectasis, fibrosis and pleural disease are some of the well known complications of the disease3,4. Patients with history of tuberculosis frequently present
with impaired lung function and a clinical picture
similar to chronic obstructive pulmonary disease (COPD)5,6. Although
treated pulmonary tuberculosis is a recognised
cause of COPD, the medical literature is scant
regarding the extent of impaired lung function7. This study is designed
to assess lung function and correlate it with medical or personal factors
that could change the outcome of the treatment of pulmonary tuberculosis
with respect to respiratory function.
METHODS
Over a period of 6 months (from January - June 1999), 46 patients with pulmonary tuberculosis who were declared cured by their treating physician were enrolled in a pulmonary out-patient clinic of Sahary Chest Hospital, Riyadh.
Fifty patients were initially registered for the study, however 4 patients were excluded because of lack of cooperation in performing spirometry tests. All these patients had pulmonary tuberculosis diagnosed by positive smear and/or culture of sputum. They were treated by standard therapy of 4 drugs (rifampicin, isoniazid, pyrazinamide or ethambutol for a minimum duration of 6 months). Because of the possible development of unrelated chest disease, five years post-treatment limit was chosen. Only patients seen within these five years were considered for the study.
Patients were subjected to spirometry (FEV1, FVC, FEV1/FVC), oxygen saturation by pulse oximetry and a posterior-anterior chest radiography. Spirometry was performed according to instructions (Schiller spirometer, Germany). Predicted values were selected appropriately for the population studied according to the current international guidelines8.
Oxygen saturation was measured by Omeda Oximeter, USA using the thumb of either hand while the patient was in the sitting position9,10. The chest radiographs were interpreted by an experienced chest physician and was compared with the previous radiographs. For a uniform computing of findings of chest x-rays a code system was developed. The interpreter of the radiographs decided which category a chest radiograph belonged to with regards to residual changes; none or mild, moderate and advanced. Mild: linear/stellate streaks, limited pleural thickening or limited scattered nodular densities; moderate: nodular dense changes, persistent cavitation, or gross pleural thickening; advanced: wide fibrotic changes, bullae or destroyed lung. Good compliance was defined as attending 90% or more of clinic appointments with drug interruption of no more than one week duration, otherwise, the patient was regarded as having poor compliance. Biodata and results of measurements were processed and coded. Analysis was done using Epi-Info and SPSS computer software and P value of < 0.05 was considered significant.
RESULTS
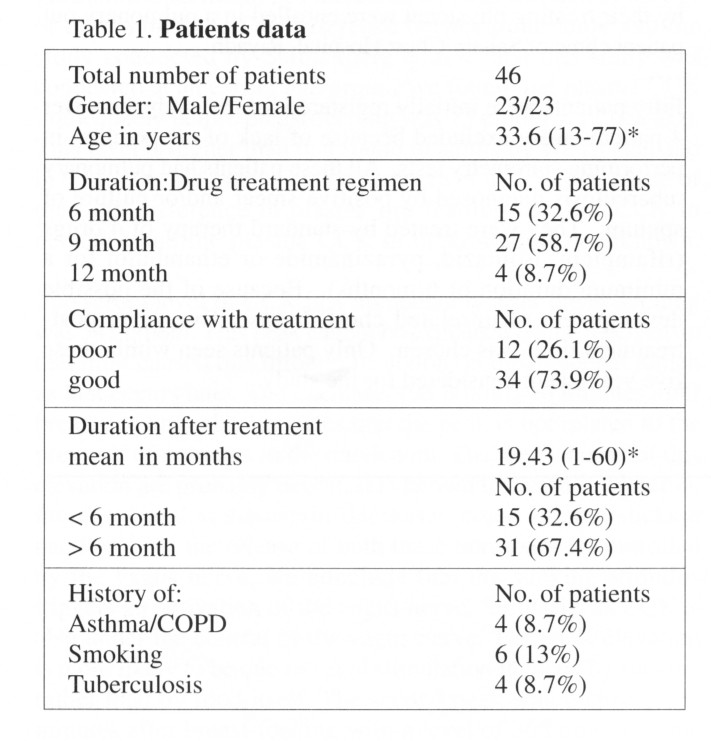
A total of 46 patients (23 males and 23 females) were studied Table 1. The duration of the treatment regimen was 6 months in 15 patients, 9 months in 27 patients and 12 months in only 4 patients. Compliance with treatment was rated by the treating physician to be good in 34 patients (73.9%) and fair or poor in 12 patients (26.1%). History of asthma or COPD was present in 4 patients (8.7%). Six patients gave history of present or past smoking (13.1%). The post treatment duration ranged between 1-60 months with a mean duration of 19.43 month (SD = 17.61). Fifteen patients (32.6%) had duration of less than 6 months and 31 patients (67.4%) had a duration of 6 months or more, up to 5 years.

Chest radiography: The plain PA chest x-rays
was clear in only 7 patients (15.2%). According to the criteria
provided in “methods” the following categories for residual changes were
found. Mild residual changes (or none) were seen in 20 patients (43.5%),
moderate residual changes were seen in 13 patients (28.3), and advanced
changes were seen in 13 patients (28.3%).
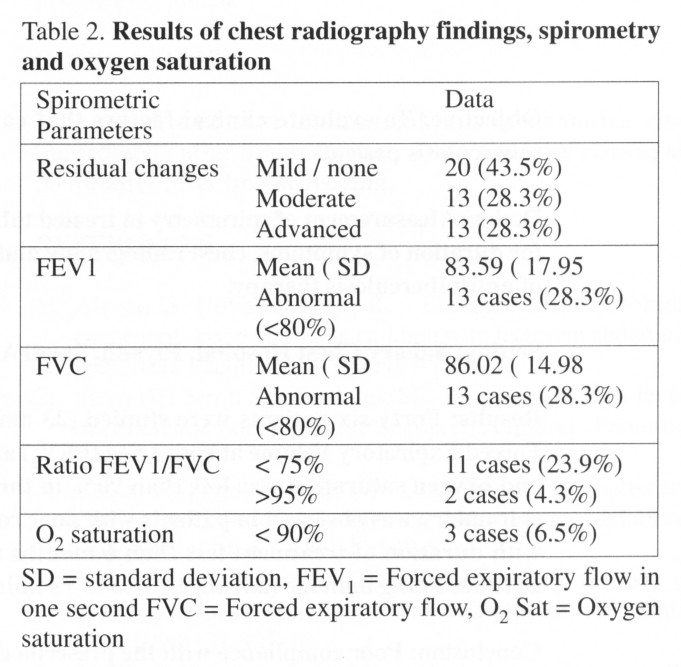
Spirometry: (Table 2) The mean percentage
of the predicted value for Forced Vital Capacity (FVC) was 86.02 (SD =
14.98). Using published criteria for assessment of airway obstruction
with a cut-off value of 80% as the lower limit of normal, 13 patients
(28.3%) were found to have abnormal FVC8. The mean percentage of
the predicted value for Forced expiratory volume in one second (FEV1) was
83.59 (SD = 17.95). Using the cut-off value as above 13 patients
(28.3%) were found to have abnormal results. The ratio of FEV1/FVC
was calculated and any results below 75% or above 95% was regarded as abnormal.
Eleven patients were found to have reduced FEV1/FVC (23.9%) indicating
an obstructive pattern and only 2 patients had ratio above 95% indicating
a restrictive pattern. All but 3 patients (6.5%) had oxygen saturation
of 90% or above as measured by finger pulse oximeter, a result that is
too small for a meaningful statistical correlation.
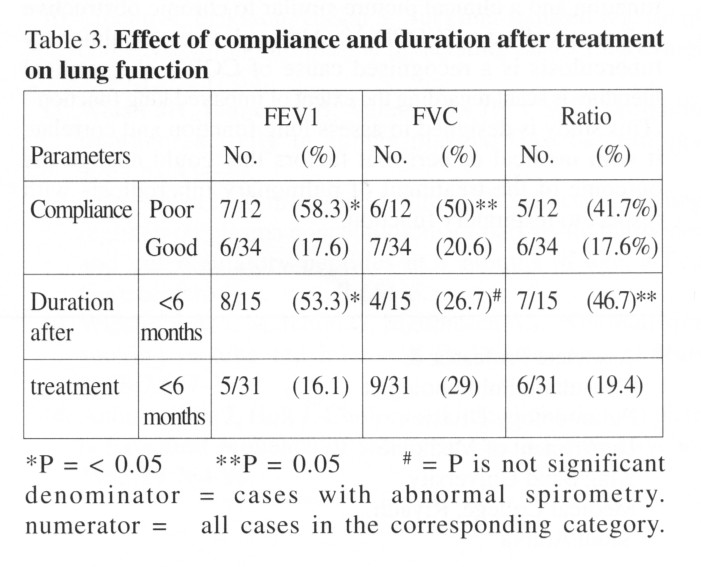
Table 3 showed the effect of compliance with
drug therapy and the effect of the post-treatment duration on the results
of spirometric measurements. More than 58% of patients with poor
compliance had abnormal FEV1, less than 80% of predicted value compared
to 17.6% of patients who complied with their drug regimen instructions.
FVC was also reduced in poorly compliant patients but results did not reach
statistical significance. A duration of 6 months or less after completion
of drug regimen for tuberculosis is associated with more cases having abnormal
FEV1 compared to a duration of 6 months or more (53.3% vs 16.1%).
Furthermore, the ratio (FEV1/FVC) was found to be abnormal in 46.7% of
cases who finished their treatment less than 6 months compared to only
19.4% for cases who finished treatment more than 6 month prior to testing,
(P = 0.05). Forced vital capacity (FVC) was not statistically different
between these two groups.
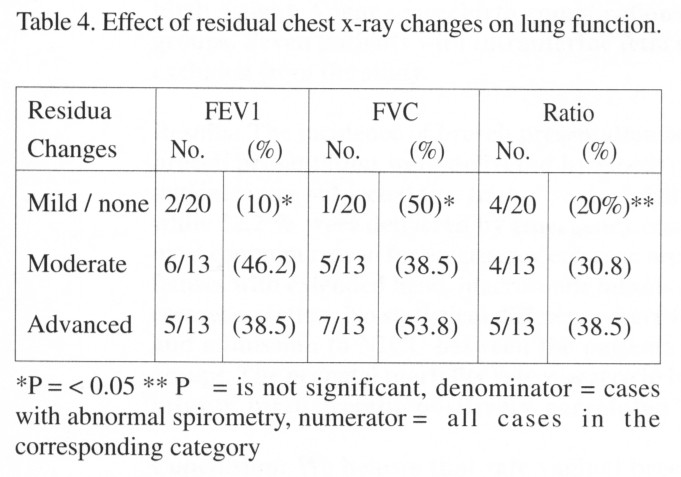
The effect of the post-tuberculosis residual changes on lung function is shown in Table 4. With normal or only mild residual changes, abnormal FEV1, FVC were seen only in 10% and 5% respectively compared to 46.2% and 38.5% for moderate changes and 38.5% and 53.8% for advanced changes. The distribution of abnormal ratio (FEV1 /FVC) among the three categories was not found to be statistically significant.
DISCUSSION
This study revealed three main factors that may affect the ventilatory function of treated tuberculosis patients: compliance with drug therapy, residual radiological changes following treatment and the length of the post-treatment period. About 26% of our patients were not fully cooperative. Compliance is a very important determinant of success of tuberculosis treatment and this study showed yet an additional reason to consolidate effort to ensure full compliance of patients with anti-tuberculosis therapy11. It is possible that irregular use or interruption of drug therapy hampers complete healing of tuberculous infection which subsequently leads to permanent parenchymal changes that reflect on lung function tests12.
Residual radiological changes in the form of pleural thickening or fibronodular changes may have been brought about by delay in diagnosis, advanced disease at the time of diagnosis or improper drug regimen13. Radiological changes seen on chest x-ray reflect parenchymal disease that gives variable impairment in lung function depending on the extent of these changes14-15. Rode and Shephard studied lung function in Canadian Innit and observed the accelerated loss of lung function in elderly patients with advanced tuberculosis16. In our study, over 56% of the cases were found to have moderate to advanced residual damage to the lungs. If future deterioration of lung function is to be avoided, much more efforts have to be directed to a good treatment program of tuberculosis management17.
Lung function seems to be affected by the duration
of the period of post therapy. The longer is the duration, the better
is the lung function. This phenomena could be explained by the possibility
that healing does continue even after stopping all anti-tuberculosis therapy
leading to further improvement of lung parenchymal structure and better
lung function measurements18-20. However, in the long term follow
up of tuberculosis patients, Vargha reported a decline of FVC of 27.7 -
54.3 ml/year and FEV1 of 28.8-35.3 ml/year over a period of 15 years21.
It is possible that the improvement observed in our study occured only
in the first few years (5 years or less) following treatment. Thereafter,
depending on the extent of the lung residual damage, patients may follow
the pattern described by Vargha as in their study no serial measurements
were done especially in the first few years following treatment for tuberculosis.
It is possible to improve the post-treatment
lung function by early diagnosis, better treatment and follow up.
It is unclear whether extending the course of drug therapy from 6 to 9
or 12 months selectively for patients presenting with an advanced parenchymal
disease would improve lung function. Further studies on this issue
are needed.
CONCLUSION
Poor compliance with the prescribed drug regimen, and significant lung damage seen on radiography were found to adversely affect the degree of loss of lung function. Furthermore, lung functions appear to improve with time, and the longer the duration of recovery from tuberculosis.
REFERENCES
1. Al-Kassimi FA, Abdullah AK, Al-Hajjaj MS, et
al. Nationwide community survey of
tuberculosis epidemiology
in Saudi Arabia. Tuber Lung Dis 1993;74:254-60.
2. Barker AF, Bardana EJ, Jr. Bronchiectasis:
update of an orphan disease. Am Rev
Respir Dis 1988;137:969-78.
3. Kolbe J, Wells AU. Bronchiectasis:
a neglected cause of respiratory morbidity and
mortality.
Respirology 1996;1:221-5.
4. Nicotra MB. Bronchiectasis.
Semin Respir Infect 1994;9:31-40.
5. Snider GL, Doctor L, Demas TA, et al.
Obstructive airway disease in patients with
treated pulmonary tuberculosis.
Am Rev Respir Dis 1971;103:625-40.
6. Bredin CP. Pulmonary function in long
term survivors of thoracoplasty. Chest
1989;95:18-20.
7. Willcox PA, Ferguson AD. Chronic Obstructive
airways disease following treated
pulmonary tuberculosis.
Respir Med 1989;83:195-8.
8. American Thoracic Society. Lung function
testing. Selection of reference values and
interpretative
strategies. Am Rev Respir Dis 1991;144:1202-18.
9. Kagle DM, Alexander CM, Berko RS, et al .
Evaluation of the Ohmeda 3700 pulse
oximeter: Steady-state
and transient response characteristics. Anesthesiology
1987;66:376-80.
10. Mengelkoch LJ, Martin D, Lawler J.
A review of the principles of pulse oximetry and
accuracy of pulse
oximeter estimates during exercise. Phys Ther 1994;74:40-9.
11. Chaulet P. Compliance with anti-tuberculosis
chemotherapy in developing countries.
Tubercle 1987;68[2
suppl]:19-24.
12. Pozcick CJ. Compliance with tuberculosis
therapy. Med Clin North Am
1993;77:1289-301.
13. Kreel L. Late complications of
tuberculosis. Postgraduate Med J 1988;64:379-81.
14. Adebo OA. Implication of
altered pulmonary function in pneumonectomy for
tuberculosis
destroyed lung. East Afr Med J 1991;68:952-8.
15. Evans SA, Turner SM, Bosch BJ, et al.
Lung function in bronchiectasis: the
influence of pseudomonas
aeruginosa. Eur Respir J 1996;9:1601-4.
16. Rode A, Shephard RJ. Lung function
in Canadian Innmit: a follow up study. Can
Med Assoc J 1984;131:741-4.
17. Bass JB Jr, Farer LS. Hopewell PC,
et al. Treatment of tuberculosis and
tuberculosis infection
in adults and children. Am J Respir Crt Care Med
1994;149:1359-74.
18. Kokkola K. Ventilatory function
and respiratory-limited exercise tolerance in
pulmonary tuberculosis.
Acta Med Scan 1973;194:169-72.
19. Skoogh BE. Lung mechanics in pulmonary
tuberculosis. I. Static lung volumes.
Scan J Respir
Dis 1973;54:148-56.
20. Lopez-Majano V. Ventilation and transfer
of gases in pulmonary tuberculosis.
Respiration
1973;30:48-63.
21. Vargha G. Fifteen year follow up of
lung function in obstructive and non-obstructive
pulmonary tuberculosis.
Acta Med Hung 1983;40:271-6.
-----------------------------------------------------------
* Associate Professor &
Consultant Pulmonologist
Pulmonology Division
Department of Medicine
King Saud University
Medical College, Riyadh
Saudi Arabia
Copyright 2001, Bahrain Medical Bulletin