with Cutaneous Granulocytic
Sarcomas
Durjoy K. Shome, MD*
Abdulla Hassan Al-Ajami, MD, FRCPA**
Shameem Sharif, MD, PhD***
Huda Jamsheer, MBChB, DCP***
Ashok K. Malik, MD, MRCPath****.
A case of agnogenic myeloid metaplasia that transformed to acute myeloblastic leukaemia terminally, is described. The acute phase was heralded by the development of cutaneous tumours, which are rarely seen in this condition.
Bahrain Med Bull 2001;23(2):87-89.
Agnogenic myeloid metaplasia (AMM), also known as primary or idiopathic myelofibrosis, is a clonal haematopoietic stem-cell disorder characterized by marrow fibrosis and extramedullary haematopoiesis. AMM is incurable, has a median survivial of 3 to 7 years, and the causes of death are mostly due to complications such as infection, haemorrhage, cardiac, hepatic or renal failure and thrombosis1,2. Conversion to acute leukaemia occurs in less than 10% of cases1,3. Skin manifestations are rare4.
We report a case of AMM that transformed to acute myeloblastic leukaemia with multiple skin tumours in the terminal phase. The case is presented in view of the rarity of the clinical presentation and the constellation of pathological findings.
THE CASE
A.H., a 43 years old man, presented in March 1998 with bone pains, slowly-growing abdominal distension, weight loss and decreasing appetite. Physical examination revealed moderate pallor, marked splenomegaly with the spleen extending below the umbilicus, and hepatomegaly 8cm below the costal margin. There was no lymphadenopathy.
A blood-count showed: haemoglobin 8.6 g/dl, platelets 382 x 109/l, and WBC 103 x 10 9/l. Peripheral blood smear showed: blasts and promyelocytes 10%, myelocytes 20%, metamyelocytes 10%, polymorphs 23% and basophils 6%. There was moderate anisopoikilocytosis, macrocytes, teardrop cells and fragmented erythrocytes. The neutrophil alkaline phosphatase score (NAP score) was normal.
Bone marrow aspiration smears showed hypercellular marrow fragments with granulocytic hyperplasia and myeloid/erythroid ratio of 20:1. Normoblastic erythroid cells and many micromegakaryocytes were seen. Differential count of marrow cells showed : blasts 6%, promyelocytes 25%, myelocytes 28%, metamyelocytes 17%, neutrophilic polymorphonuclears 25%, basophils 4% lymyhocytes 12%, plasma cells 2% and normoblasts 4%.
The trephine biopsy however, showed markedly thickened
bone trabeculae with constricted marrow spaces showing marked fibroblastic
proliferation (Fig. 1). Marrow reticulin.was markedly increased. Liver
biopsy showed extensive intrasinusoidal haematopoiesis. Biochemical tests
showed mild hypoalbuminemia (32 g/l), hyperglobulinemia (40 g/l) and hyperuricemia
(526 /mM).

Cytogenetic study of unstimulated marrow aspirate showed normal chromosome complement and banding pattern in 25 metaphases. In situ hybridisation with probes for the BCR and ABL loci showed these were spatially separated with no fusion, confirming Ph1-negative status.
The patient was started on hydroxyurea 1.5 g daily. Over the next few months, WBC counts were controlled at a dose of 2 g daily. Eight months later, the patient was splenectomised in view of repeated blood transfusion requirement and pressure symptoms due to the massive spleen. The specimen of spleen was bulky and weighed 3.3 Kg. The cut surface showed markedly thickened capsule. Histologically, the entire organ was extensively infiltrated by myeloid cells at different stages of development and dysplastic megakaryocytes that were present in clusters (Fig.2). Erythroid precursors were less prominent and were usually present in sinusoids. Extensive fibrosis, deposition of hemosiderin pigment and a few atrophic lymphoid follicles were also seen.

In October 1999, a-interferron was started but had to be stopped two months later because of troublesome flu-like symptoms. In December 1999, multiple skin nodules developed all over his body measuring 1-2 cm. in diameter. Fine-needle aspiration of these nodules showed evidence of extramedullary hematopoiesis with immature myeloid cells, megakaryocytes and normoblasts (Fig.3). These nodules increased in size and some became painful. The largest of these (Fig.4), over the right scapular region had to be treated with radiotherapy (300 Gy/15 hours over 3 weeks), and showed complete response.


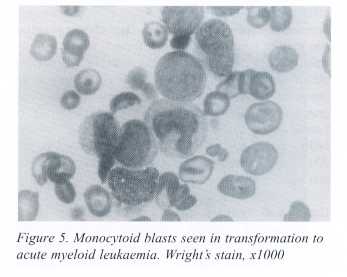
At this time, a marrow aspirate yielded a dry tap while the biopsy showed no hemopoietic activity and reticulin fibrosis. In March 2000 the patient was hospitalized with fever, generalized lymphadenopathy and signs and symptoms of raised intracranial tension. Opthalmoscopic examination showed bilateral papilledema and preretinal haemorrhages. CSF examination showed a WBC count of 1500 /mm3 with a majority of blast cells. Bone marrow aspiration smears showed 48% blasts and 8% promyelocytes with reduced megakaryocytes and erythroid cells. Blasts were monocytoid (Fig. 5), and showed moderate nonspecific esterase activity. FNAC of a skin nodule also showed numerous blast cells. A diagnosis of transformation of AMM to acute myeloblastic leukemia was made.
Chemotherapy with high-dose cytarabine 3 g/m2 BID on days 1,3,5,7 and idarubicin 12 mg/m2 daily for 3 days) and intrathecal triple therapy (methotrexate, cytarabine and hydrocortisone) was started. However the patient’s condition continued to worsen and he died shortly thereafter.
DISCUSSION
AMM is a relatively uncommon disorder in Bahrain. In an analysis of 213 haematological malignancies diagnosed between 1986 to 1995 at Salmaniya, only 3 cases were detected5.
In AMM, extramedullary haematopoietic foci can cause organ enlargement or tumour formation in almost any tissue. Common sites are spleen liver, lymph nodes, kidney and adrenal glands. Less often involved are bowel, breast, lungs pleura, mediastinum, mesentery, skin, synovium, thymus, lower urinary tract, ovaries and intra-cranial or intra-spinal epidural space 1,2,6. In this case histological evidence of such haematopoiesis was seen in the liver biopsy and spleen, with skin involvement in the later stages. Our patient showed several poor prognostic features at presentation: anaemia, high WBC count and high numbers of circulating blasts. Presence of hepatomegaly is a debatable indicator of poor prognosis in AMM7.
Skin involvement in AMM may occur in the form
of erythematous plaques, nodules, diffuse or papular erythema, ulcers or
bullae 4. Transition to acute leukaemia preceded by the development of
granulocytic sarcomas, as seen in this case, is a rare event 8, 9.
Another notable feature was that transformation
to myeloblastic leukaemia occurred simultaneously in the marrow and cutaneous
tumours along with CNS involvement.
Clonality studies in AMM have shown that the trilineage proliferation (myeloid, erythroid and megakaryocytic) which occurs in varying degrees, is actually monoclonal and is due to involvement at stem cell level 7. Fibroblastic proliferation with collagen deposition, often the most prominent feature in the marrow, is a secondary reaction 10. Fibrosis is induced by cytokines released by the proliferating hemopoietic cells, principally megakaryocytes 10 that are prominently seen in fibrotic areas. Increased marrow collagen may be due to both decreased breakdown and increased synthesis of the protein. Degradation of collagen may be retarded by platelet factor 4, which inhibits collagenase 11. Among factors that stimulate fibrosis are platelet-derived growth factor (PDGF) and calmodulin (stimulate fibroblast proliferation) and transforming growth factor b (TGF-b, increases collagen synthesis) 7, 12. Levels of both PDGF and TGF-b are increased in circulating megakaryocyte fragments and platelets in AMM 13. TGF-b may be the principal mediator of collagen accumulation in AMM 12.
Understanding of these pathogenetic mechanisms forms the rationale of treatment with agents that suppress the action TGF-b (Interferon-a, suramin) or with anti-proliferative action on megakaryocytes (Vitamin D analogs, anagrelide) 7, 12. Interferon-a may ameliorate bone pain, splenomegaly and thrombocytopenia but its use is limited by its side-effects such as flu-like symptoms and worsening anemia 14, 15. In the present case too, Interferon-a was used, but could not be adequately tried because of flu-like symptoms.
CONCLUSION
Cutaneous tumours (granulocytic sarcomas) developing in AMM is a rare and ominous sign and may precede acute leukaemic transformation of the disease. This should alert the clinician to investigate the marrow and suspicions extramedullary sites for such transformation of the disease.
REFERENCES
1. Clark DA, Williams WL. Myelofibrosis.
In: Lee GR, Foerster J,
Lukens J, et al,
eds. Wintrobe’s Clinical Hematology. 10th ed. Baltimore:
Williams &
Wilkins, 1999:2390-404.
2. Lichtman MA. Agnogenic Myeloid
Metaplasia. In: William WJ, Beutler
E, Erslev AJ,
Lichtman MA, eds. Hematology 4th ed, New York: McGraw-
Hill,1990:
225.
3. Silverstein MN, Brown AL, Linman
JW. Idiopathic Myeloid Metaplasia –
its evolution
into acute leukemia. Arch Intern Med 1973;132:709-712.
4. Loewy G, Mathew A, Distenfeld
A. Skin manifestation of agnogenic
myeloid metaplasia.
AM J Hematol 1994; 45: 167-70.
5. Dash S, Al-Hilli F. Haematological
malignancies in Bahrain. Bahrain Med
Bull 1998; 20:33-37.
6. Ward HP, Block MH. The natural
history of agnogenic myeloid metaplasia
(AMM) and a critical
evaluation of its relationship with the
myeloproliferative
syndrome. Medicine 1971; 50: 357-420.
7. Tefferi A. Myelofibrosis with myeloid
metaplasia. N Eng J Med
2000;342:1255-1265.
8. Fayemi AO, Gerber MA, Cohen
I et al. Myeloid Sarcoma. Cancer 1973;
32:253.
9. Jacobs P, Sellars S. Granulocytic
sarcoma preceding leukaemic transformation
in
myelofibrosis. Postgrad Med J 1985;61:1069.
10. Reilly JT. Pathogenesis of idiopathic
myelofibrosis: present status and future
directions. Br
J Haematol 1994;88:1-8.
11. Castro-Malspina H. Pathogenesis of
myelofibrosis. Prog Clin Biol Res
1984;154:427-454.
12. Clark DA, Williams WL. Myelofibrosis.
In: Lee GR, Foerster J, Lukens J,
Paraskevas
F, Greer JP, Rodgers GM, eds. Wintrobe’s Clinical Hematology 10th
ed., Baltimore,
Williams & Wilkins, 1999: 2390-2404.
13. Martyre MC, Magdelenat H, Bryckaert
MC, Laine-Bidron C, Calvo F. Increased
intraplatelet
levels of platelet-derived growth factor and transforming growth
factor-beta
in patients with myelofibrosis with myeloid metaplasia. Br J
Haematol
1991;77:80-86.
14. McCarthy D, Clark J, Giles F. The treatment
of myelofibrosis with alpha-
interferon.
Br J Haematol 1991;78:590-591.
15. List AF, Doll DC. Alpha-interferon in the
treatment of idiopathic myelofibrosis.
Br J Haematol
(letter)1992;80:566-567.
--------------------------------------------------------------------------------------------------------
* Consultant,
Haematology and Blood Bank
*** Consultant, Cytology
**** Consultant, Histopathology
Department of Pathology.
** Consultant Haematologist
and Oncologist
Department of Medicine.
Salmaniya Medical Complex
State of Bahrain

