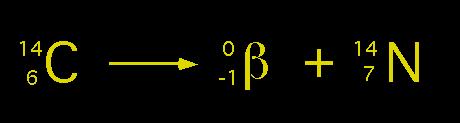Otzi the iceman, the world's oldest mummy, made headlines when he was discovered in 1991 by hikers along the border of Austria and Italy.
HOW OLD IS OUR PAL OTZI?

Well, that question has been answered by carbon dating Otzi’s remains.
This leads us to our question:
HOW DOES CARBON DATING WORK?
By Jason and Andrea
__________________________________________________
Absolute dating using radiocarbon is the most common technique in archeology that is employed to determine the age of a sample containing carbon.

In order to understand this process, it is critical to know that:
Carbon is an atom having 6 protons and 6 neutrons and that Carbon 14 is an unstable radioactive isotope of Carbon, thereby having a different number of neutrons.

Only two of the Carbon Isotopes are stable, C-12 & C-13. All other Carbon Isotopes are unstable and they degrade into something else. Only C-12, C-13, and C-14 exist naturally in our environment. The other isotopes must be produced.
The farther away the Mass Number gets from 12 &13, the faster the isotopes degrade. The Blue Numbers in the chart indicate half-lives, the time it takes for one half of the atoms in a sample to decay to a new substance.
Each different isotope has a different half-life, but the half-life of each specific isotope stays constant, and as far as we have observed, it never changes. For Uranium 238 it is always 4,500,000,000 years. For Carbon 14 it is always 5730 years. For Carbon 15 it is always 2.25 seconds, etc.
__________________________________________________
The Breakdown Of Radioactive Atoms Is A Self-Corrective Process!
When Carbons 9, 10 & 11 breakdown they release a positron which effectively turns a proton into a neutron.
The opposite occurs with Carbons 14, 15 & 16. They release a Beta Particle when they breakdown, which effectively converts a neutron into a proton.
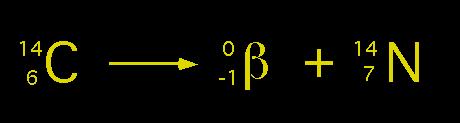
(A BALANCED EQUATION FOR - CARBON 14 EMITTING A BETA PARTICLE)
__________________________________________________
Carbon 14 is found on earth because it is continuously produced in our upper atmosphere and becomes bound into carbon dioxide molecules in our atmosphere.

This happens because cosmic rays cause some of the atoms in the upper atmosphere to fly apart into pieces. Neutrons that come from these fragmented molecules run into other molecules. When a neutron collides into a Nitrogen 14 atom, it becomes Carbon 14.

Two other reactions produce Carbon 14, but in much smaller yields.
Carbon 13 reacting with with Oxygen 17 and with He 4.
__________________________________________________
The production and degradation of Carbon 14 is going on at the same rate.

Because Carbon 14 gets bound into carbon dioxide molecules in the atmosphere it is absorbed into plant life. These plants are consumed by animals. All the food that we eat is contaminated with the same level of Carbon 14, so, essentially, the whole Biosphere contains Carbon 14 at the same concentration.

As long as an organism is alive, C-14 will be taken in. When the animal or organism dies, this process is disrupted. The remaining C-14 continues to decay, reverting back to Nitrogen, at a constant, measurable rate.

Because we have determined that the half-life of C-14 is 5730 years, all a scientist would have to do is determine how many half-lives the loss represents. By measuring the amount of C-14 left in the tissue, such as bone, an archeologist can arrive at the date of the sample, the point at which the organism stopped absorbing C-14.

The ratio of Carbon 12 to Carbon 14 is 848 billion to 1.
The amount of Carbon 14 present in a sample is compared in proportion to the amount of Carbon 12 in the same sample. Determining the difference of these amounts is the key to determining how long the organism has been dead because the difference represents the loss in Carbon 14 the organism experienced over time.

With the nuclear accelerator mass spectrometry technique, which directly counts Carbon 14 atoms, it is possible for us to detect samples that have undergone as many as 13 half-lives (75,000 years) of Carbon 14 degradation.

Our pal Otzi’s death has been carbon dated at approximately 5,300 years ago. That’s even older than Holly!
__________________________________________________
REFERENCES:
Matthews, Luke. “Periodizing the Past.” General Anthropology Handout 9b
Brown, Michael. “Carbon 14 Dating.”
17 Nov. 2003 {http://www.creation-science-prophecy.com/C14fp.htm}
"The Iceman 'Thaweth'". National Geographic World.
March 2001. National Geographic Society.