THE
JAR TEST
People
all over the world depend on water treatment plants (WTPs) to provide them
with safe,
clean
drinking water. As such, water treatment and the methods it entails
have been around for many years. Before the water can be sent out
to the public, it is necessary to be able to determine if the water is
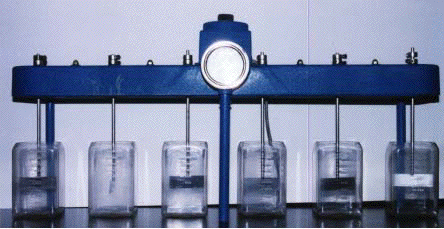
clean enough for public use. But how is this done? The jar
test is just one of many methods that can be employed to assess the cleanliness
of a water sample. By measuring turbidity and monitoring pH, the
technicians at a water treatment plant can determine whether or not the
water is safe.
In this laboratory, we performed the jar test on twelve different samples taken from the Delaware River near Easton, Pennsylvania. By adding varying amounts of alum and buffer, and measuring intial and final pH values, conductivity, temperature, and turbidity, we were able to look at trends in the data to see just what the most effective approach to water treatment is.
This
laboratory is a follow-up to the lab of the previous week, where we visited
the Easton Water Treatment Plant. On this page you will find links
to related topics, pictures from the Jar Test Laboratory, and most importantly,
our
lab
write-up !!
![]()
Links:
Laboratory
7- The Jar Test
by
Michelle DiMeglio and Katrina Gibbons
EPA
Announces Arsenic Standard For Drinking Water of 10 Parts per Billion
![]()