Light Problems
Introduction
c = speed of light = 3.00 x 108 m s-1
h = Planck's Constant = 6.63 x 10-34 J sec
E = Energy ( unit is Joules)
f = frequency ( cycles/s, Hertz, Hz,
1/s, s-1)
 = lambda = wavelength ( meters,
centimeters, nanometers) = lambda = wavelength ( meters,
centimeters, nanometers)
| 1. |
 |
Frequency and Wavelength are inversely related. If one increases the other
must decrease.
|
| 2. |
E = hf |
Frequency and Energy are directly related. If one increases the other must
also increase.
|
| 3. |
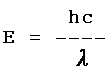
|
Energy and Wavelength are inversely related. If one increases the other
must decrease.
|
|
Problem Solving Steps
|
| 1. |
Identify the known and unknown
variables. Remember - h and c are always known terms.
|
| 2. |
Pick the equation that has as its only
unknown term, the term that is your unknown.
|
| 3. |
Substitute the values and units into the
equation.
|
| 4. |
Plug values into the calculator and cancel
units. Round off to the appropriate number of
significant figures. |
Table of Contents
Light Problems
1. If light has a wavelength of 5.32 x 10-7
m, what is the
frequency ?
2. If light has a frequency of 8.34 x 1014 Hz, what is the
wavelength ?
3. If light has a frequency of 1.57 x 1016 Hz, what is the
energy ?
4. If light has an energy of 4.33 x 10-19 Joules, what is the
frequency ?
5. If light has an energy of 8.21 x 10-20 Joules, what is the
wavelength ?
6. If light has a wavelength of 3.92 x 10-8
m, what is the
energy ?
Table of Contents
Light Answers
| 1. |
If light has a wavelength of 5.32 x 10-7
m, what is the
frequency ?
|
|
 = 5.32 x 10 -7
m
c = 3.00 x 10 8 m s -1
= 5.32 x 10 -7
m
c = 3.00 x 10 8 m s -1
f =
? 
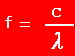 3.00 x 10 8 m s -1
3.00 x 10 8 m s -1
= ------------------------
5.32 x 10 -7
m
f = 5.64 x 10 16 s -1 |
| |
|
|
|
| 2. |
If light has a frequency of 8.34 x 1014 Hz, what is the
wavelength ?
|
|
f
= 8.34 x 10 14 s -1
c = 3.00 x 10 8 m s -1
 =
? =
? 
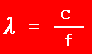 3.00 x 10 8 m s -1
3.00 x 10 8 m s -1
= ------------------------
8.34 x
10 14 s -1
 =
3.60 x 10 -7 m =
3.60 x 10 -7 m
|
| |
|
|
|
| 3. |
If light has a frequency of 1.57 x 1016 Hz, what is the
energy ?
|
|
f = 1.57 x 1016
s -1
h = 6.63 x 10 -34 J
s c = 3.00 x 10 8
m s -1
E =
?
E = hf
E = 6.63 x 10 -34 J s x 1.57
x 1016 s -1
E = 1.04 x 10 -17 J |
| |
|
|
|
| 4. |
If light has an energy of 4.33 x 10-19
J, what is the
frequency ?
|
|
E = 4.33 x 10
-19 J
h = 6.63 x 10 -34 J
s c = 3.00 x 10 8
m s -1
f =
?
E = hf
E 4.33 x 10 -19
J
f = ----- = ----------------------- = 6.53 x 10 14 s -1
h 6.63 x 10 -34
J s |
| |
|
|
|
| 5. |
If light has an energy of 8.21 x 10-20 Joules, what is the
wavelength ?
|
|
E = 8.21 x 10
-20 J
h = 6.63 x 10 -34 J
s c = 3.00 x 10 8
m s -1
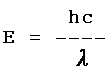 => =>

6.63
x 10 -34 J s x 3.00 x 10 8 m s -1
 =
-------------------------------------------------- =
--------------------------------------------------
8.21 x 10 -20 J
 =
2.42 x 10 -6 m =
2.42 x 10 -6 m |
| |
|
|
|
| 6. |
If light has a wavelength of 3.92 x 10-8 m, what is the
energy ?
|
|
 = 3.92 x 10 -8 m
= 3.92 x 10 -8 m
h = 6.63 x 10 -34 J
s c = 3.00 x 10 8
m s -1
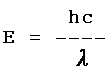
6.63
x 10 -34 J s x 3.00 x 10 8 m s -1
E = ---------------------------------------------------
3.92 x 10 -8 m
E = 5.07 x 10 -18 J |
Table of Contents
|