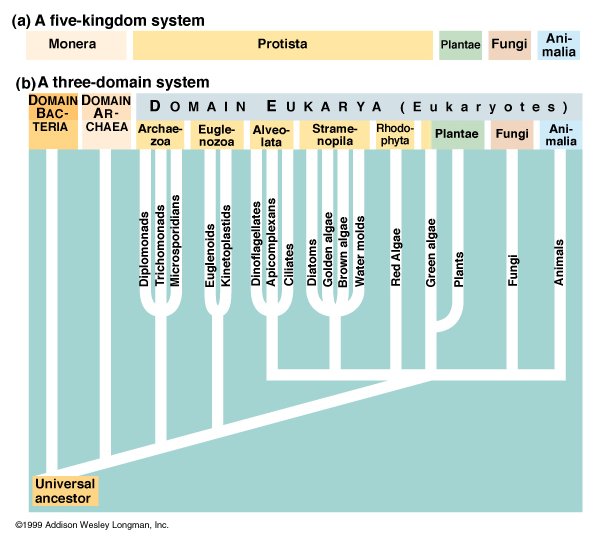

The five-kingdom system, first proposed in 1969, placed organisms whose cells lack a nucleus (prokaryotes) in the kingdom Monera, while all other living things were classified into four kingdoms: protoctista (formerly known as protists), plants, fungi and animals. Thanks to the pioneering work of Dr. Carl Woese, biologists now realise that the differences between various kinds of prokaryotes dwarf those between the other four kingdoms. Prokaryotes are now placed in two distinct domains: (eu)bacteria and archaea. Archaea look like bacteria but are fundamentally different on a molecular level. They are found in extreme environments, which require methanogenic, halophilic, or thermophilic metabolisms. All other organisms are placed in the domain of eukaryotes. However, the differences between various kinds of protoctista are much greater than those between plants, fungi and animals. Despite being superseded by recent research, the five-kingdom system is still widely used by biologists.
Organisms in this kingdom are prokaryotes - a term which includes bacteria and archaea - while organisms in the other kingdoms (animals, fungi, plants and protoctista) are classified as eukaryotes.
There are fundamental differences between eukaryotes and prokaryotes at the cellular level. Eukaryotic cells possess an extra level of complexity which prokaryotes lack: they contain specialised structures called organelles. Eukaryotic cells also have a central compartment or nucleus, where the DNA is stored in paired chromosomes. Prokaryotic cells lack a nucleus - their DNA may be contained in a single circular chromosome or in a long strand. Prokaryotes are thus the simplest cellular organisms, from a structural perspective.
Bacteria are the best-studied prokaryotes. They are usually considered to possess a simple behavioural repertoire, but as the following quote from Di Primio, Muller and Lengeler (2000) shows, they are capable of a diverse range of sensory, motor and communicative feats:
1. Bacteria have internal and external sensors of different types.
2. Bacteria can synthesize sensors and effectors when required and eliminate them when no longer needed (a solution in response to changes of the environment less frequently found in higher organisms). [N.B. "Effector" is defined by A Dictionary of Science (1999) as "a cell or organ that produces a physiological response when stimulated by a nerve impulse" - V.T.]
3. Bacteria have been able to move for about 3 billion years now by means of rotating effectors (flagella) that act like a ship's screw...
4. Bacteria react to stimuli in indirect ways and the coupling between stimuli and responses is modifiable.
5. Bacteria identify and compare stimuli at different times (a process based on sensory stimulation and a simple memory).
6. Bacteria are able to integrate different (e.g. positive and negative) stimuli when given simultaneously.
7. Bacteria show purposeful (goal-oriented) behavior...
8. They communicate by means of pheromones (signaling molecules) and by exchanging genetic information (quasi-sexual behavior).
9. They co-operate and compete in both an intra- and interspecific way (i.e. with bacteria of the same and of other species) Di Primio, Muller and Lengeler (2000, pp. 4 - 7, italics mine).
The kingdom of protoctista includes single-celled microbes with nuclei, as well as certain multi-celled organisms, such as kelp (which can reach up to 100 metres long), that do not belong to the plant, animal or fungi kingdoms. Amoebae, algae, seaweeds, slime moulds, ciliates, diatoms, paramecia and forams belong in this kingdom.
The first thing that needs to be said about protoctista is that they are eukaryotes, whereas bacteria are prokaryotes. Briefly, eukaryotic cells have a nucleus, while prokaryotic cells lack one. Although protoctista are commonly lumped together with bacteria and viruses as "microbes", they are actually much more like plants, animals and fungi, at a cellular level.
It now appears that most of the biological diversity within the eukaryotes lies among the protoctista, and many scientists believe that it is just as inappropriate to lump all protoctista into a single kingdom as it was to group all prokaryotes together in a single domain. (Prokaryotes are now classified into two domains: bacteria and archaea.)
There are fundamental differences between eukaryotes and prokaryotes at the cellular level. Eukaryotic cells possess an extra level of complexity which prokaryotes lack: they contain specialised structures called organelles. Eukaryotic cells have a nucleus, where the DNA is stored in paired chromosomes. Prokaryotic cells lack a nucleus - their DNA is usually contained in a single circular chromosome.
Eukaryotes share anatomical similarities which make their information transfer pathways more complex as well as faster than those of prokaryotes. The processes whereby materials (e.g. nutrients) and information are diffused within a eukaryotic cell are more complex than those in a prokaryotic cell, simply because eukaryotic cells are so much bigger (Kaiser, 2001; Illingworth, 1999; Cotterill, 2001, p. 5). Because a prokaryotic cell is so small, it has a large surface-to-volume ratio, so nutrients can rapidly reach any part of the cell's interior. Eukaryotic cells, being much larger, have a smaller surface-to-volume ratio, so nutrients cannot rapidly spread to all internal parts of the cell. For this reason, eukaryotic cells require a variety of specialised structures to carry out metabolism, provide energy, and transport chemicals throughout the cell.
Additionally, all eukaryotes (protoctista, plants, fungi and animals) make use of rapid electrochemical signalling to transmit information, in addition to the slow process of chemical diffusion used by bacteria. Eukaryotic cells are much larger than bacterial cells, so they are able to detect tiny spatial concentration gradients from one side of the cell to another when searching for food, instead of relying on measurements taken at successive 3-second intervals as bacteria do. Unlike bacteria, eukaryotes know which way to swim, but they use larger motors and a different internal signalling system. For example, electrochemical signalling (resulting from the interaction of proteins with calcium ions and the energy-storing molecule ATP) enables protozoa to move around.

There is currently some disagreement on what defines a plant. Many lay-people would say "photosynthesis", but there are bacteria (cyanobacteria) which photosynthesise and there are some plants (beech drops) which do not. The old saw, "That which is green is a plant", falls foul of counter-instances, too - a few plants have lost their green pigment during their evolution, and the status of green algae as plants is in dispute. However, plants do have certain common features: they are eukaryotes, and they develop from embryos - multicellular structures enclosed in maternal tissue. Because all plants develop from embryos, they are all multicellular.
Cell walls are a feature of plants (as well as bacteria, fungi and algae) but not animals. Many protoctista also lack them. Plant cell walls play a major role in the structure and support of a plant. The cell walls of plants are made of cellulose. Different authorities define plants on the basis of either chemistry (restricting plants to the groups where the cells contain a membrane-bounded chloroplast, where the products of photosynthesis are stored, with a particular type of chlorophyll) or the structure of the wall of the chloroplast. For this reason, the status of certain algae (e.g. red algae) as plants remains controversial (Hoverkamp, 2002; McCourt, Chapman, Buchheim and Mishler, 1995; World Biodiversity Project, 2000).

Fungi were once considered to be plants, but are now classified in a separate kingdom. Fungi are thought to closer to animals than any other kingdom.
The living body of a fungus, known as the mycelium, is made out of a web of tiny filaments called hyphae. The mycelium is usually hidden in the soil, in wood, or another food source. The part of the fungus that we see is only the ?gfruit?h of the organism.
Most fungi build their cell walls out of chitin - a material which is also found in the hard outer shells of insects and other arthropods. Plants do not make chitin.
Fungi do not possess stomachs, but feed by absorbing nutrients from the organic material in which they live. They must digest their food before it can pass through the cell wall into the hyphae. Hyphae secrete acids and enzymes that break the surrounding organic material down into simple molecules they can easily absorb (Fun Facts about Fungi, 1998).
Animals are often defined as organisms that are:
a morula, followed bya blastula (a multicellular embryo that develops from the zygote produced by fertilization of a large egg by a smaller sperm), and finally
a gastrula (a hollow sac that forms the embryonic precursor to the digestive tract, by means of which animals ingest nutrients and excrete waste)
(World Biodiversity Database, 2000).
This definition is both too broad and too narrow. Many other kinds of eukaryotic organisms are also multicellular - e.g. plants, fungi and slime moulds. Fungi and many protoctista are also heterotrophic, while plants develop from embryos that result from the sexual fusion of a sperm and an egg. These features, then, are not unique to animals. On the other hand, development from a blastula is unique to animals, but a few animals (certain sponges) do not develop in this way (World Biodiversity Database, 2000).
The only feature that appears unique to animals is: a special kind of extracellular matrix (ECM - the substance between cells) that is composed of four types of molecules - collagen, proteoglycans, adhesive glycoproteins, and integrin - that are created inside, but exist outside, the cells of animals. Extracellular matrix plays a key role in the development of animals. It guides the development of mobile cells in the developing embryo, and also helps to control the transition of cells from one type to another. (Mobile cells enable animals to develop from single cells into working individuals composed of many cells.) It appears that all animals share this complex pattern of development mediated by their extracellular matrix, whereas all other multicellular organisms do not (Morris, 1993).
Animals exhibit different kinds of sensory behaviour, such as attraction to light, avoidance of noxious chemicals, and the ability to sense dissolved gases and temperature. Such behaviour is found in members of all five kingdoms of living things, but animals have most elaborated this theme (World Biodiversity Database, 2000). Generally, animals' senses can be classified into three groups: electromagnetic senses (which detect changes in light, heat, and other kinds of electromagnetic radiation); mechanical senses (which respond to sound, touch, gravity, and stretching); and chemical senses (taste and smell).
There is a fundamental difference between Aristotle's teleological definition of animals, and the contemporary scientific definition. For Aristotle, as we have seen, the existence of sensory capacities was a defining characteristic of animals: without these discriminatory abilities, animals could not survive, avoid danger or acquire what they need (De Anima 3.9, 432a16, 3.12, 434a30 - 434b3). Locomotion was a sufficient but not a necessary condition for being an animal (De Anima 3.9, 432a16; 3.9, 432b19-20, 3.12, 434b8).
By contrast, the modern scientific definition of "animal" is based on the fundamental similarities in their structure and bio-molecules - especially at the cellular level - between organisms that were formerly classified as animals on the basis of their sensory capacities. It is at the cellular level that animals look most alike (Morris, 1993). Because the criterion that defines animals is now based on their biochemistry and not their psychology, certain organisms that might not have been previously regarded as animals (owing to their lack of perception) can now be classified as animals, while organisms whose biochemistry is too different from that of animals can be relegated to another kingdom, sensory or locomotive capacities notwithstanding.
Case study: the lysis-lysogeny decision in viruses

Viruses (and all other organisms) exhibit some degree of phenotypic plasticity, which can be defined as the ability of organisms with the same genotype to vary their developmental pattern, phenotype or behaviour in response to varying environmental conditions (Ancel and Fontana, 2002).
A well-known case of phenotypic plasticity in viruses is the lysis-lysogeny decision, whereby parasitic lambda-phage viruses adopt a bet-hedging strategy in order to cope with fluctuations in the availability of their hosts (E. coli bacteria). When a virus invades a host bacterium, it may kill the host immediately by multiplying until the host's cell walls burst (lysis) or it remain quiescent and may confer immunity to infection upon its host (lysogeny). The important thing is that the actual decision to invade or lie low is a random one, which depends entirely on thermal background noise. Preuss (2000) describes the process thus:
When a bacteriophage ("bacteria eater") virus injects its own dna (sic) into a microorganism such as Escherichia coli, the host cell apparatus rapidly expresses the program on the viral dna (sic) that decides whether or not to kill the host immediately. Under conditions that are less than optimal for replication, the phage may actually confer immunity to further infection upon the host (lysogeny). But if conditions are good, the virus produces so many copies of itself that the cell walls burst - a state known as lysis - and the infection spreads.Two independently produced regulatory proteins compete to control whether the invading genes will remain quiescent or be expressed. Because of inescapable thermal noise, the outcome in any given case is random, and the proportion of the population in either state changes according to conditions such as cell nutrition and the number of invading particles per cell...
Arkin and his colleagues have found that the underlying stochastic [i.e. random - V.J.T] mechanisms of the lysis-lysogeny decision circuit... depend entirely upon the chance timing and concentrations of bursts of competing proteins that act to reinforce or inhibit one another.
"...Thermal fluctuation at the molecular level makes for diversity in cells that start out under identical conditions," says Arkin. "The phage actually makes use of noise as a survival mechanism: sometimes it pays to multiply and infect as many hosts as possible, sometimes it pays to lie low. Either way, the viral population is prepared to cope with changing conditions" (italics mine).
If we simply defined a sensor as "a device that responds to a physical stimulus", as some popular Web-based definitions do (e.g. http://www.campbellsci.com/glossary.html, http://www.allaboutmems.com/glossary.html) then we would have to conclude from the above description that viruses possess sensors. But in fact, viruses, which are little more than living molecules, have no built-in sensors and possess no information that enables them to realise their goals. Thus their behaviour, when making the lysis-lysogeny decision, does not even meet the criteria required for the adoption of Dennett's intentional stance. The viruses do not possess (i.e. encode or store) information about environmental conditions conducive to replication, but simply respond to changing conditions in a random, non-specific manner. To exclude this case, we need a more rigorous definition of sensor, stipulating that the response must be stimulus-specific.
Case study 2: bacteria
Bacteria, unlike viruses, certainly possess sensors. According to John S. Parkinson, a professor of biology at the University of Utah, "most organisms - even bacteria - can sense sound, light, pressure, gravity and chemicals" (University of Utah, 2002). E. coli bacteria "can sense and respond to changes in temperature, osmolarity, pH, noxious chemicals, DNA-damaging agents, mineral abundance, energy sources, electron acceptors, metabolites, chemical signals from other bacteria, and parasites" (Meyers and Bull, 2002, p. 555). Bacteria are very sensitive to chemicals - for instance, E. coli bacteria have five different kinds of sensors which they use to detect food. As Di Primio, Muller and Lengeler (2000, pp. 4 - 5) explain, common bacteria like E. coli swim in chemical gradients towards attractants (e.g. glucose) or away from repellents (e.g. benzoate) - a phenomenon known as chemotaxis. Other bacteria display phototaxis and magnetotaxis, or movement in response to light and magnetic fields, respectively (Martin and Gordon, 2001, p. 219). Bacteria possess an elaborate chemosensory signaling pathway, which involves the phosphorylation (combination with phosphorus compounds) of a set of proteins in the cytoplasm of a bacterial cell (Blair, 1995, p. 489).
Bacteria possess specialised "receptors" or information-encoding devices, which are sensitive to light, chemicals, magnetic fields and so on. These receptors may or may not be activated, depending on the local environment. A bacterium has two kinds of motion: directed movement (a "run", which occurs when a bacterium's rotary motors or flagella, rotate in a counter-clockwise direction) and random tumbling (which occurs when a bacterium's flagella suddenly change direction and rotate clockwise). When the external section of a bacterial receptor recognizes and binds its target, a signal passes through the rest of the receptor and causes sequential changes in two proteins inside the bacterium. (This two-protein sensing system is found in all bacteria and in many other life-forms, but not in animals.) The first protein is called a kinase and sits next to the receptor. Normally, when there is no signal, this protein activates a second protein, the regulator, which interacts with the gear shift of a bacterium's flagella, causing them to turn clockwise and the bacterium to tumble randomly, about once every second. However, when there is a signal from the receptor, the kinase cannot activate the regulator protein. Thus, the flagella continue to turn counterclockwise, and the bacterium, instead of tumbling, swims smoothly towards the target (Aegerter, 1997). What is more, these receptors can even store information about their objects over a short period of time - in other words, they possess a kind of "memory" (to be discussed later).
As we saw in Part B, Section 1, Aristotle maintained that a sensory capacity was more than a mere capacity to respond to environmental changes. Two other criteria were required: reception of form without matter and the existence of a mean. I interpreted these conditions to mean that an organism had to be able to encode information (form without matter) about environmental changes, within an organ which could exist in different information "states" (allowing it to serve as a "mean" between them).
On Aristotle's account, there appears to be no good reason for denying sensory perception to bacteria, as their receptors can encode information about their objects (attractants and repellents), at different actualisations (i.e. concentrations of attractants), spanning five orders of magnitude (Illingworth, 1999).
This may be a surprising conclusion, given Aristotle's denial that plants can perceive (De Anima 2.3, 414a31, 3.13, 435b1). However, knowing what we now do about organisms, it is reasonable to infer that he would revise his conclusions. A modern commentator, Charles Kahn, has argued that Aristotle "would have been obliged to "grant one-celled animals "a share in perception proper... since the possession of a sense faculty is included in the definition of an animal" (1979, p. 25). Moreover, Aristotle would have probably classified bacteria as animals, since they are capable of locomotion, which he regarded as a capacity possessed only by some animals (De Anima 3.9, 432b19) In fact, Aristotle explicitly declared: "No non-stationary body has a soul without perception...If, then, any body that travels did not have perception, it would be destroyed... After all how is it to be nourished?" (De Anima 3.12, 434b2-3, 434a33, 434b1). It should also be pointed out that van Leeuwenhoek, the first person to observe bacteria through his microscope, called them "little animals" or "animalcules" (Waggoner, 1996).
Cotterill (2001) denies the existence of proper senses in bacteria, because the order of stimulus and response is reversed: instead of environmental changes acting as the stimulus which causes a motor response in a bacterium, the bacterium initiates its own random tumbling movements and thereby gains information about its surroundings, using a short-term memory that informs it as to whether the concentrations of certain molecules in its environment have changed in the last few seconds. As Cotterill puts it:
The stimulus in this case is thus the motor movement, while the response is that of the impinging substances. This is just the opposite of a reflex... There are no senses, of the type found in more advanced species, and the internal state of the creature is embodied in the concentrations of various molecules. These concentrations dictate the creature's movements (2001, pp. 3-4).
In a creature with reflexes, by contrast, the motor response is "independent of the creature's internal state" (2001, p. 5), and the reaction of a specialised receptor cell in the creature's body to "an unprovoked stimulus" (2001, p. 5, italics mine) leads to a rapid, automatic motor response.
Cotterill's main argument can be recast in an Aristotelian form. He acknowledges that bacteria can store information relating to the concentrations of substances in their surroundings (2001, p. 6), but insists that sensing an object means something more than that. In Aristotle's account, sensing an object meant being affected by it in a certain way, and an animal's desire of the sensed object produces locomotion towards it (De Anima 3.10, 433a16). In bacteria, according to Cotterrill, "sensing" an object means acting upon it, and it is the perpetual movement (locomotion) of bacteria that enables them to "sense" chemicals. That is, locomotion is prior to "sensation".
We can express Cotterill's objection another way, by saying that sensations are a form of "feedback", whereas bacteria seem to use "feed-forward" instead to navigate around their environment. Certainly, bacteria are much more active in probing their environment than other sensitive organisms, because of their size: since they are too small to gauge spatial variations in the concentrations of molecules in their environment (e.g. differences between concentrations at their anterior and posterior extremities), they have to actively "sample" their surroundings, relying on a form of short-term chemical memory (discussed in section 2) to alert them to changes.
However, we need to distinguish between two kinds of bacterial motion: (a) the random tumbling movements which bacteria initiate in order to probe their surroundings, and (b) the directed "runs" which they make along chemical gradients towards attractants. I would agree with Cotterill's characterisation of movement of the former kind as a stimulus and its feedback about its environment as a response. But it is only movement of the latter kind which Aristotle would characterise as locomotion, or "movement started by the object of desire" (De Anima 3.10, 433a16). This kind of motion is subsequent to, not prior to, the act of sensing the attractant. Moreover, the change in a bacterium's pattern of movement (from random tumbling to directed swimming) is produced by a combination of events occurring both inside and outside its body: its internally driven propulsion which allows it to probe its environment, and the variations in the concentrations of attractant or repellent molecules in its environment. This does not sound so different from the Aristotelian notion of perception as "being affected in a certain way" (De Anima 2.11, 424a1).
Having said that, I think Cotterill (2001) has done a valuable job in highlighting some philosophically significant differences between different grades of sensitivity.
Below, I discuss four major evolutionary milestones highlighted by Cotterill, discuss the different meanings of "sense" and highlight the implications for intentional agency.
1.3.1 Evolutionary milestone one: the appearance of electrochemical signalling in eukaryotes
Case study 1: Protoctista
Sensory phenomena such as chemotaxis, thermotaxis (movement in response to heat), phototaxis, geotaxis (movement in response to gravity) and an ability to identify suitable mates are well-attested for protoctista (Martin and Gordon, 2001, p. 409). As we saw above, many of these capacities are also found in bacteria. There are, however, some important differences between bacteria and protoctista which determine the way they sense objects in their environment.
Protoctista, unlike bacteria, are eukaryotes. There are anatomical similarities shared by all eukaryotes which make their information transfer pathways more complex as well as faster than those of prokaryotes. Eukaryotic cells not only contain a nucleus, but are also about 10 times larger than bacterial cells. For that reason alone, the processes whereby materials (e.g. nutrients) and information are diffused within a eukaryotic cell are more complex than those in a prokaryotic cell (Kaiser, 2001; Illingworth, 1999; Cotterill, 2001, p. 5). Because a prokaryotic cell is so small, it has a large surface-to-volume ratio, so nutrients can rapidly reach any part of the cell's interior. Since a eukaryotic cell is much larger than a prokaryotic cell, it has a smaller surface-to-volume ratio, so nutrients cannot rapidly spread to all internal parts of the cell. Also, the communication systems between computational elements that work in prokaryotes may or may not be appropriate for eukaryotes.
A motile eukaryotic cell also travels much faster than a prokaryotic cell (e.g. a bacterium), and therefore encounters danger or opportunity far more frequently. It needs a way to communicate rapidly between the forward end of the cell and the flagella which usually propel it from the rear.
Chemical signals, whether transmitted by diffusion or circulation, do not move fast enough from one end of a eukaryotic cell to another to meet its needs for rapid communication of information. Accordingly, all eukaryotes (protoctista, plants, fungi and animals) make use of rapid electrochemical signalling to transmit information, in addition to the slow process of chemical diffusion used by bacteria. For example, electrochemical signalling (resulting from the interaction of proteins with calcium ions and the energy-storing molecule ATP) enables protozoa to move around.
Scientists attempting to trace the evolution of sensory capacities in eukaryotes often focus on those of modern-day single-celled microbes such as Paramecium, the amoeba Dictyostelium and Euglena gracilis. Electrochemical signalling has been identified in these creatures, using chemicals similar to those found in the nervous systems of vertebrates.
Despite these differences, Cotterill (2001, p. 5) does not regard protoctista as possessing "what we would call senses", as the main practical difference with bacteria is simply the speed-up of information transfer.
There is, however, one other difference between senses in bacteria and protoctista which is philosophically relevant, as it pertains to the definition of a sense. Whereas in bacteria, as we have seen, locomotion is prior to "sensation", one might argue that in eukaryotes it is the other way round: information received by these organisms' sensors makes them move towards their goals. Whereas bacteria rely on measurements taken at successive 3-second intervals to find food when probing their surroundings, eukaryotic cells, which are much larger than bacterial cells, are able to detect tiny spatial concentration gradients from one side of the cell to another when searching for food. It thus seems that locomotion in eukaryotes is caused by changes that they sense in their surroundings.
However, the distinction between prokaryotes and eukaryotes, as regards the cause of their locomotion, is not a clearcut one: one-celled eukaryotes, like bacteria, propel themselves by means of their flagella (long whip-like structures used in cellular locomotion). These eukaryotic cells thus obtain sensory information about their surroundings as a result of their own movements.
From the foregoing account, the differences between the sensory capacities of bacteria and the simplest eukaryotes (protoctista) appear to be quantitative rather than qualitative.
Case study 2: Plants

Plants possess an impressive range of sensory capacities, which have been described in a recent report in "New Scientist" (Coghlan, 26 September 1998). The following are the key points:
- Research on a humble weed known as thale cress (Arabidopsis thaliana, a plant with a relatively simple genome) reveals that plants can "see". They have proteins attached to light-sensitive compounds. Proteins called phytochromes enable plants to work out the quality of light and compete with neighbouring plants. Other proteins called crytochromes enable plants to work out whether it is night or day, the length of the day, the quantity of light, and the direction it is coming from.
- A particular gene in Arabidopsis allows its roots to "taste" the soil and find out where vital nutrients are most abundant, thereby saving energy, as the roots grow towards the source of the nutrients, rather than randomly. Additionally, an enzyme called apyrase, found on root surfaces, allows various plants to "taste" ATP, a useful source of short-term energy. The leaves of corn, beet and cotton plants can also "taste" the saliva of caterpillars and respond by secreting volatile compounds which attract parasitic wasps, which kill the caterpillars.
- Tomato plants exude a substance called methyl jasmonate when wounded. When neighbouring plants "smell" this signal, they prepare for battle by producing chemicals that repel insects or attract predators. Recent research also suggests that plants can smell smoke, and that this triggers forest generation after a fire, as buried seeds germinate.
- The responsiveness of certain plants (the Venus fly trap, or Mimosa) to touch is well-known, but these abilities are simply amplifications of what all plants can do, according to researchers. In particular, plants respond to the buffeting of the wind by strengthening tissues that are being swayed. Within a tenth of a second after being pushed around, calcium ions flood into the plants' cellular fluid, and activate genes that strengthen their cell walls.
- Some plants even respond to sound. Prolonged exposure to 2 kilohertz frequencies (about the same as a human voice), at 70 to 80 decibels (a bit louder than speaking) can double the growth rate of dwarf pea plants and quadruple the germination rate of radishes. (However, talking to plants will not work: "You'd have to sit there and talk to them for days", says one researcher.) It is believed that sound induces the biosynthesis of gibberellic acid, which increases growth and germination. Inhibiting the synthesis of this hormone blocks the enhancing effects of sound.
What the above research shows is that plants have a wide range of discriminatory abilities that allow them to fine-tune their responses to their environment. Should these abilities be called senses? In Part B, Section 1, I examined Aristotle's argument that a sensory capacity should not be defined merely as a capacity to respond to environmental changes. Aristotle's defining criteria (reception of form without matter and the existence of a mean) were interpreted to mean that an organism with sensory capacities had to be able to encode information (form without matter) about environmental changes, within an organ which could exist in different information "states" (allowing it to serve as a "mean" between them).
It will be recalled that for Aristotle, the existence of sensory perception (aisthesis) was both a necessary and a sufficient criterion for being an animal, while locomotion was a sufficient but not a necessary criterion. Although Aristotle denied perception to plants (De Anima 2.3, 414a31; 3.13, 435b1), it is by no means clear from his own definition of sense (discussed in Part B, Section 1) why the discriminatory abilities of some plants (e.g. thale cress, discussed above, which uses proteins to identify the quality of light, as well as whether it is night or day, the length of the day, the quantity of light, and the direction it is coming from) are fundamentally different from the sensory capacities of animals.
Nevertheless, there are some important features which plants lack. Because plants, unlike most animals, do not possess nerve cells, nervous systems or reflexes, their behavioural repertoire is limited, and their motor responses are simply a function of their internal state (Cotterill, 2001, p. 5).
1.3.2 Evolutionary milestones two, three and four: nervous systems, movement mediators and reflexes
Case study 1: Cnidaria

Nerve cells are only found in animals. In fact, they are unique to so-called "true" animals (the subkingdom Eumetazoa, which excludes sponges). The simplest of these "true" animals are the Cnidaria - commonly known as coelenterates, including animals such as jellyfish, sea anemones, corals and freshwater hydra, which possess the most rudimentary nervous systems found in nature (World Biodiversity Database, 2000). Animals with nerve cells are thought to have appeared 700 million years ago. Cotterill describes the transition:
Instead of merely being deployed on the organism's bonding surface, the receptor molecules became incorporated in the membranes of the multicellular creature's specialized receptor cells (2001, p. 5).
Most jellyfish exhibit a sluggish response to stimuli, and their behavioural repertoire is not significantly different from "lower" life-forms (Cotterill, 2001, p. 5; Prescott, 2001, pp. 5-6). However, two types of jellyfish, Aglantha digitale and Amphogona apicicata, exhibit dual reponse patterns, mediated by two different types of nerve impulses: a slow feeding mode, and a fast reaction mode which enables these jellyfish to rapidly escape from predators.
To Cotterill, the distinctive fast reaction mode in these jellyfish suggests the presence of two components which serve to distinguish what he considers to be true senses from those found in "lower" organisms:
(i) a neuron-based movement mediator which is capable of activating different motor programs in response to environmental feedback; and(ii) a genuine autonomous reflex, characterised by "a receptor cell's reaction to an unprovoked stimulus leading unaided to a motor response" (2001, p. 5). For Cotterill, the most significant feature of this response is that it is independent of the creature's internal state.
Recent research (Martin, 2000) suggests that some cnidarians may have much more sophisticated senses than previously suspected. Cubozoans, or killer box jellyfish, are known to have complex eyes, similar in their basic design to those of vertebrates, despite the absence of a brain or central nervous system. The eyes connect into the neural network of the jellyfish, and there is evidence that they can see images. It has been suggested that for cubozoans, vision may play a role in feeding and reproductive behaviour:
Certain cubozoans are know to chase small fish and seize them with their tentacles. Further, many cubozoans exhibit complex sexual behaviors in which the males chase the females, grasp them with a tentacle, and subsequently inject packets of sperm into them. Vision may be important to the jellyfish for such complex behaviors (Martin, 2000).
While the occurrence of reflexes and the sense of vision in jellyfish is still the subject of vigorous debate, the occurrence of reflexes and bona fide senses in other, more "advanced" phyla of animals (e.g. arthropods, molluscs and annelids) is not in doubt (Cotterill, 2001, p. 5).
Case study 2: Aplysia

The aquatic mollusc Aplysia exhibits a further evolutionary innovation which goes beyond even the most advanced cnidaria: a rudimentary form of sensory processor. The chief advantage of a sensory processor is that it enables an animal's nervous system to find correlations between different sensory inputs, these being tied to motor output after undergoing some intermediate processing (Cotterill, 2001, pp. 5-6). Although its entire nervous system consists of only a few hundred neurons, Aplysia is capable of associative learning (i.e. classical conditioning as well as instrumental conditioning). I will discuss learning in section 4.
Case study: bacteria

The simplest kind of memory found in organisms is a kind of chemical memory found in bacteria, which use it to search for food. Because bacteria are too small to detect any changes in the concentration of nutrients from one end of their one-celled bodies to the other, their only way of deciding which way to move in their search for food is to rely on a very short-term memory mechanism, in which they move around randomly, sample the concentrations of chemicals in their environment at regular short intervals, and compare the current concentration of attractant chemicals in their environment with the concentration during the last measurement. If there is an increase in the concentration of attractants, the bacteria will keep moving in the same direction. In other words, bacteria use temporal gradients rather than spatial gradients to detect food.
The following account of the simple memory mechanism used by bacteria is pooled from a variety of sources (Illingworth, 1999; Di Primio, Muller and Lengeler, 2000, pp. 4 - 6; Cotterill, 2001, pp. 3-5; University of Utah, 2002).
A bacterial cell is extremely sensitive: it can sense a chemical if even one of its sensors comes into contact with a chemical, and it can detect the change if the number of sensors in contact with a chemical increases by just one. A bacterium has sensors known as methyl-accepting chemically sensitive proteins, or MCPs, on its surface. Thre are four varieties of MCPs, allowing bacteria to track different attractants at once. MCPs generate large output signals (via a protein inside the cell, called Che A) in response to any changes that occur. The chemical sensors in a bacterial cell signal changes in the attractant concentrations, rather than absolute concentrations of the attractants. These signals alter the cell's motion.
A bacterium's memory is a consequence of the fact that its tracking system takes a few seconds to catch up with any alteration in chemical concentrations, enabling the bacterial cell to compare its present state with its state a short time ago. The number of receptors stimulated by attractive or repellent molecules (apparently this number is an average of measurements taken over a period of about one second) is "compared" with the number of receptors stimulated in the previous measurement (stored as an internal signal representing the average of measurements taken 3 seconds ago). The memory possessed by bacteria is minimal: it can store just one set of intermediate results, allowing bacteria to remember any changes in the concentration of attractant chemicals that have occurred in the past 3 seconds.
The memory possessed by bacteria gives them the appearance of purposeful movement. As we have seen, bacteria have two kinds of motion: directed movement (a "run") and random tumbling. When the concentration of an attractant increases over time, sensors send chemical signals via messenger proteins within the cell, to the cell's flagella (propellers). The bacteria reduce the frequency of their random tumbling motion and prolong the directed "run" motion of their flagella, enabling them to maintain their direction of motion and keep moving towards the attractant. (There is no point in bacteria swimming in the same direction for any longer, because random Brownian motion will knock them off course anyway.) Repellents have the opposite effect: bacteria respond by increasing their random tumbling motion until the concentration starts to decrease, which triggers a "run" away from the repellent.
As the concentration of an attractant increases, the affinity of a bacterial cell's chemically sensitive proteins (MCPs) for attractants decreases, as a result of a chemical change called methylation. The cell becomes less responsive to the attractant - in other words, sensitized.
One could suppose that the bacteria are exhibiting "purposeful movement" and are searching for food, on the basis of what they remember. There are two good reasons for rejecting this interpretation.
First, the sole warrant for saying that bacteria move purposefully is that their behaviour possesses a kind of finality - in this case, intrinsic finality, as they are alive. And while the chemical basis of a bacterium's memory does not preclude it from being a mental state - our own memory has a chemical basis - the "memory" exhibited by a bacterium can be simply defined by the quantity and quality of substances currently inside the cell, some of which persist for a short time, even after the division of the cell (Kilian and Muller, 2001, p. 2). A richer, mentalistic account appears redundant.
Second, the three-second memory exhibited by a bacterium is severely limited:
... the creature's recent history ... determines the instantaneous magnitudes of the various molecular concentrations. No chemical record is kept of the magnitudes of the various concentrations at different times... [T]he information in its environment concerning the spatial distribution of nutrients... is merely lumped into a single number, and the bacterium's cognitive repertoire is telescoped into a single binary choice, clockwise or anti-clockwise rotation of the flagellum (Cotterill, 2001, p. 22).
Bacterial memory is constrained in three significant ways, since it is:
- binary (the bacterium simply decides whether to continue tumbling randomly or keep moving in its present direction),
- relative (the bacterium does not remember absolute concentrations of attractants, but changes in concentrations) and
- ahistorical (no chemical record is kept of the magnitudes of the various concentrations at different times - instead, the bacterium simply compares its present circumstances with its situation a few seconds earlier).
Roediger, Marsh and Lee (2002) provide a useful summary of the current literature relating to memory. The distinction proposed by Ryle in 1949 between declarative memory and procedural memory - roughly, between "knowing that" and "knowing how" - is still widely invoked, although some recent authors refer to the latter simply as non-declarative memory. Tulving (1972) has suggested that there are two forms of declarative memory: an episodic memory for particular events (e.g. "Where did you go on vacation last summer?"), which involves accessing the time and place of their occurrence, and a semantic memory for general facts about the world (e.g. zebras have four legs). The distinctions are not as clearcut as one would like: it seems that most if not all memory tasks have some procedural component, although some procedural tasks (e.g. tying one's shoe-laces) require no declarative component (Roediger, Marsh and Lee, 2002, pp. 5-6).
In evolutionary terms, procedural memory is thought to be the oldest and is shared in some form by virtually all animals (Tulving, 1985), although I have not been able to locate any evidence for its occurrence in sponges. If, like some authors (Roediger, Marsh and Lee, 2002), we choose to regard classical conditioning and motor skill learning as the most primitive forms of procedural memory, then its occurrence in cnidaria also appears unlikely, for reasons that will become apparent in section 4.
Although procedural memory is commonly held to include capacities such as classical conditioning, motor skill learning and complex (skill-based) problem-solving (Roediger, Marsh and Lee, 2002, p. 5), the term "procedural memory" remains poorly defined in the literature, as the following selection of definitions illustrates:
Table - Some popular Web-based definitions of procedural memory
|
The "lowest" form of memory in which simple associations between stimuli and responses are formed (Enders Tulving, world-renowned expert on memory). (Web source: http://citl.tamu.edu/citl-glossary-main.htm) |
|
Knowledge of "how", i.e., of skills, either perceptual-motor or cognitive (University of North Carolina at Chapel Hill, Glossary of terms for Cognitive Psychology [Psychology 020] course taught by Adjunct Professor Dr. Gordon Pitz in 2002). (Web source: http://www.unc.edu/courses/pre2000fall/psyc020/) |
|
Memory for motor movements and skills (Benjamin B. Lahey, Psychology: An Introduction, 7th edition, McGraw-Hill, p. 241). (Web source: http://www.mhhe.com/socscience/intro/cafe/lahey7/student/olc/chap07glossary.mhtml) |
|
Memory for skills and habits, such as riding a bike or hitting a baseball, sometimes referred to as "nondeclarative memory" (Robert S. Feldman, Essentials of Understanding Psychology, 5th edition, University of Massachusetts, Amherst, p. 241). (Web source: http://highered.mcgraw-hill.com/sites/0072494263/student_view0/chapter6/glossary.html) |
The most common item listed in the above definitions is "memory for skills", but the term "skill" is vague: does it refer to motor skills, perceptual skills, cognitive skills, or perhaps all three? Memory for habits is also listed in one definition as a form of procedural memory. While there is general agreement (Roediger, Marsh and Lee, 2002, pp. 5-6) that any organism undergoing classical conditioning acquires a new skill, the foregoing definitions are ambiguous as to whether habituation should count as the acquisition of a habit. (I discuss classical conditioning at further length in section 4 of part B.)
Finally, psychologists often distinguish between different forms of memory according to how long the memory trace lasts. Terms such as "working memory" and "long-term memory" are commonly used, but there is as yet no generally accepted theoretical framework for explaining how these forms of memory actually work (Roediger, Marsh and Lee, 2002).
Most definitions of procedural memory do not include habituation, although on one of the definitions in the table above, it might count. In any case, there are no credible reports in the literature of habituation in bacteria or archaea, let alone classical conditioning. (Although Di Primio, Muller and Lengeler (2000, p. 7) claim that habituation occurs in all cellular organisms, even bacteria, the evidence cited in favour of this sweeping claim is the observation that following prolonged exposure to an attractant, bacteria change from a "run" to a "tumble" movement. However, if we examine the chemical basis for so-called "habituation" in bacteria, it appears to be a case of sensory adaptation - a phenomenon in which an organism's response to a stimulus may diminish because the organism's sensory organs no longer detect it - rather than habituation, where the response to a stimulus wanes because the experience of it is repeated over and over again. I discuss this point at further length in section 4.)
We may therefore safely conclude that prokaryotes do not possess any kind of procedural memory.
There have been claims that plants and protoctista are capable of undergoing classical conditioning (see Hennessey et al., 1979, pp. 417-423; Abramson et al., 2002, pp. 175-176), which would imply that they possess a primitive form of procedural memory. I discuss these claims below in section 4, where I conclude that they have yet to be satisfactorily demonstrated (in the case of plants) or replicated (in the case of protoctista). For the time being, it seems prudent to conclude that classical conditioning is confined to animals with central nervous systems.
Worms thus appear to be the most "primitive" creatures with a rudimentary form of procedural memory.
The assessment of declarative memory in non-human animals is a challenging task, as they cannot verbally declare what they remember. The procedure normally used by scientists is the delayed non-matching to sample (DNMS) tasks, in which animals are presented with a sample object and then after a delay interval are asked to choose between the sample object and a novel object. Choosing the novel, non-matching object is the correct choice. The ability of primates, rats, pigeons (Young and Wasserman, 2001, http://www.pigeon.psy.tufts.edu/avc/young/) and even honeybees (Giurfa et al., 2001, http://iibce.edu.uy/semineuro/giurfa20nat2001.pdf) to perform these tasks seems to indicate that they possess some form of semantic memory.
In work involving the more traditional simultaneous MTS [match-to-sample] or NMTS tasks, ravens and gulls (Benjamini, 1983), and jackdaws, jays, and rooks (Wilson, Mackintosh, & Boakes, 1985) have all been documented to learn to choose the stimulus that matches the sample and to generalize this learning to novel stimuli. Furthermore, Pepperberg (1987) has trained a language-trained grey parrot (Alex) to identify the specific difference between objects (matter, shape, or color). Alex learned to properly identify the dimension on which the objects differed at well above chance levels and to generalize this knowledge to novel objects (Young and Wasserman, 2001, http://www.pigeon.psy.tufts.edu/avc/young/conclusions.html).
Episodic memory is considered to be the most recent in evolutionary terms, and its occurrence in non-human animals is still disputed. There is evidence that western scrub jays possess an episodic-like memory, but at the present time, the alternative hypothesis that some form of semantic memory can account for the birds' behaviour cannot be ruled out (Shettleworth, 2001; Clayton et al., 2003; Emery and Clayton, 2004).
Case study: indirect, modifiable stimulus-response coupling in bacteria

Di Primio, Muller and Lengeler (2000, pp. 4, 7) argue that the occurrence of indirect, modifiable stimulus-response coupling in bacteria is evidence of primitive cognition on their part.
The coupling between stimulus and response in bacteria is indirect because when a sensor detects a chemical, it activates a chain of chemical reactions, each of which is reversible. The coupling is modifiable: if E. coli's sensors detect an attractant (e.g. galactose), and later sense another compound (e.g. glucose) that is more attractive than the first one, a "weighing" of the relative quality of the nutrients occurs, and the chain of reactions resulting in directed motion is amplified. The co-presence of attractants and repellents in solution generates an integration of the "run" and "tumble" responses, at the chemical level (so-called "conflict resolution").
However, as Kilian and Muller (2001) point out, the way in which bacteria react to a chemical is utterly inflexible, at the molecular level:
In unicellulars, in each of the molecules of an information transfer path, regardless out of how many elements it is composed, both a functional specifity (sic) and a goal specifity (sic) can be discerned. Each molecule contacts its goal substrates and interacts with them according to its respective function. Both specifities (sic) are pregiven in the enzymatic active center on the molecular level (2001, p. 3).
In other words, the apparently complex behaviour of bacteria in response to multiple simultaneous stimuli (positive and/or negative) is merely the resultant of two or more inflexible existing patterns of behaviour. The rules governing the behaviour of bacteria do not change; all that changes are the external circumstances (i.e. the presence of a new attractant or repellent). The behaviour of the bacteria can be perfectly well described using a mind-neutral goal-centred intentional stance. E. coli bacteria have a built-in preference for one goal (glucose) over another (galactose), which explains their response to the new information that glucose is nearby. There is no need to invoke mental states here.
In short: even behaviour that is categorised as "modifiable" may be the result of an underlying fixed action pattern. (Mathematically, adding or removing a stimulus can be represented as changing the value of an input variable for one of the functions determining the value of the output.)
The mere fact that the output variable z of a function F has different values under different circumstances does not imply that z's behaviour is flexible, as the following mathematical example shows.
Suppose that we can describe a piece of behaviour in an organism using a mathematical function F and some input variables (or parameters) x1, x2, x3, ... xN, where the value of the output variable z is F(x1, x2, x3, ... xN). The above definition entails that even when the values of x1, x2, x3, ... xN vary over time, the behaviour still conforms to a fixed pattern, so long as the function F remains the same.
Nevertheless, some authors (Godfrey-Smith, 2001; Carruthers, 2004) refer to this kind of output variability as "flexible" behaviour, as the following three examples illustrate.
Case study 1: The lac operon system in bacteria
Godfrey-Smith (2001, p. 6) claims that "plants and bacteria do exhibit some capacities for flexible response to environmental conditions, using environmental cues to control development and metabolism." As an example, he cites the lac operon system in E. coli bacteria:
These bacteria can respond to a change in local food type through processes in which the availability of a nutrient affects the regulation of genes which code for enzymes able to digest that nutrient.
From the foregoing description, it should be clear that we are not dealing with flexible behaviour here, but with behaviour which can be described by a fixed mathematical function, whose input variables are the local concentrations of different nutrients (lactose and galactose).
Case study 2: Plants' ability to adjust to changes in lighting
Another example of flexibility cited by Godfrey-Smith is the ability of plants to adjust to different lighting conditions:
For example, many plants can determine not just that they are being shaded, but that they are being shaded by other plants. This is done by detecting the wavelength properties of the reflected light. The plants respond to shading by growing longer shoots... (2001, p. 7).Here, the length of the shoots can be represented as an invariant (fixed) mathematical function of the wavelength(s) of the incoming light.
Case study 3: Singing strategies in crickets
Carruthers (2004), while rejecting (rightly in my view) a mentalistic interpretation of the singing behaviour of crickets, describes it as flexible. According to my definition, such behaviour is fixed:
It turns out that even flexibility of behavioral strategy isn't really sufficient for a creature to count as having a mind, indeed. For innate behavioral programs can have a conditional format. It used to be thought, for example, that all male crickets sing to attract mates. But this isn't so; and for good reason. For singing exposes crickets to predation, and also makes them targets for parasitic flies who drop their eggs on them. Many male crickets adopt the alternative strategy of waiting silently as a satellite of a singing male, and intercepting and attempting to mate with any females attracted by the song. But the two different strategies aren't fixed. A previously silent male may begin to sing if one or more of the singing males is removed... (2004)
Here, the output behaviour for a male cricket (wait or sing) can be described as an invariant function of two variables: (a) its "attractiveness rating" and (b) the number of attractive males nearby.
One surprising implication of my definition of a fixed pattern of behaviour is that even in a fixed pattern, the value of the output variable z may be determined by two or more different functions, depending on the values of the inputs. A simple case would be the following program statement, written in Pascal code:
IF (x > 4) THEN z := F(x)
ELSE
BEGINIF (x > -2) THEN z:= (F(x) + G(x))END;
ELSE z := G(x)
In the list of functions, we might define F(x) as, say, x + 3 and G(x) as (x - 5) / 2. Here we have two functions being invoked for different values of the input variable x, but the value of the output variable z remains the same for the same value(s) of the input(s). If the value of x changes from 5 to -7, the function determining the value of the variable z changes from F(x) to G(x), but because the program has not changed, we can still describe the overall pattern as fixed.
In the case of the Pascal statement above, a change in
(i) the IF statement conditions (e.g. from (x > 4) to (x > 5)) orwould qualify as an instance of flexible behaviour.
(ii) the definition of the functions F or G, or
(iii) the number of parameters they require,
In other words, truly flexible behaviour requires not just new values of the output variable for different values of the inputs, but new patterns of output, new kinds of input, or new conditions under which the output patterns are generated.
Bacteria, like other organisms, display an impressive repertoire of adaptive behaviour in response to environmental changes. Some of this behaviour qualifies as flexible, according to the definition given above. I propose to examine three aspects of their behaviour - cellular regulation, phenotypic plasticity and gene-swapping - which best exemplify their internal dynamism, and discuss the issue of whether any of them are flexible enough to qualify as evidence for mental states in bacteria.
3.4.1 Cellular regulation in bacteria
Cellular regulation in bacteria is governed by a complex network of interactions between biomolecules and structures inside each cell. Wolf and Arkin (2003) describe how this network can be simplified by identifying recurring regulatory motifs - small regulatory subnetworks that can be classified according to their function, architecture or dynamics:
Regulatory motifs proposed to date ... include switches, amplitude filters, oscillators, frequency filters, noise filters and amplifiers, combinatorial logic, homeostats, rheostats, logic gates and memory elements... (2003, pp. 125-126).
According to Wolf and Arkin (2003), bacteria exhibit a wide variety of motifs which regulate cell activity. Regulatory switches allow bacterial cells to make an all-or-nothing (on-off) response to internal or external signals. Some cellular switches (like door-bells) lack a memory; others (like light switches) remember their setting. Bistable switches in bacterial cells have a memory and exhibit a history dependence (known as hysteresis): their pattern of responding to variations in the strength of a signal depends on the initial setting of the switch, and they tend to react slowly to changes in the signal value. There is one pattern of responding when the signal increases in strength (a "going-up" pattern), and another when it decreases (a "coming-down" pattern). In each pattern, the switch tends to remain in its current setting until the signal reaches a certain level that makes it suddenly "flip" settings, over a narrow range. These two patterns make up what is called a hysteresis loop - a switching pattern commonly found in ferromagnetic materials. Bistable switches can be set to an "on" or "off" position indefinitely by an environmental stimulus encountered by the cell.
Certainly, the behaviour of a bistable switch can be described using Dennett's intentional stance. It remembers its setting, it resists changes and "tries" to "ignore" random noise within the cell that would cause it to flip continually backwards and forwards between on and off states. (This conservative trait is adaptive, as it cuts out disruptive interference.) Most impressively, it is apparently capable of flexibility: it can change from one pattern of responding (the "going-up" pattern) to another (the "going-down" pattern). Does this change of patterns qualify as flexible behaviour, according to our definition? I would argue that it does not. In fact, the switch is inflexible within a limited range of input signal values, as it resists changes to its setting. Although we can speak of the switch as remembering its old setting, it would be wrong to say that it changes its pattern as its setting fluctuates. I would argue that instead of saying that the switch acquires a new pattern as its setting fluctuates, we could more economically describe the "going-up" and "coming-down" patterns as part of a single pattern (the hysteresis loop) which is built into the chemistry of the switch. The value of the output ("on" or "off") can be defined a function of two variables: the strength of the current input signal and that of the previous input signal. Together, these two pieces of information tell us whether the signal is "going-up" or "coming-down". Hysteresis in bacterial cells is a time-lag phenomenon, rather than a true case of flexible behaviour.
Another motif in bacterial cells is the biphasic amplitude filter, in which a device amplifies an input signal only if it falls within a specific range. Mathematically, this can be described as invoking a function F to convert an input signal into a new output signal, if and only if the input signal falls within a certain range. A cellular process controlled by the filter can only be initiated by a particular set of circumstances in the cell's environment or within the cell itself. This is a very selective way of responding to environmental inputs, but it cannot be termed flexible, as there is no change in the pattern of response over time.
The same can be said for bandpass frequency filters, which allow bacterial cells to function in a noisy environment by filtering signals within a frequency-domain, extracting information from them and separating them into their component parts. Bandpass filters amplify a signal if and only if it oscillates at a particular frequency. Again, we cannot speak of flexible behaviour here, as the cell does not change its pattern of responding to signals over time.
Other motifs describe the way in which cellular processes, which have to be regulated with precision, are protected from disruptive background noise arising from protein translation. Mechanisms such as negative feedback, redundancy, biochemical cascades, checkpoints and delay elements, as well as frequency filters, serve to ensure that cells can maintain their normal routines. Other motifs actually exploit noise, either by harnessing it to drive an ordered process or using it to amplify a signal. However complex and sophisticated these mechanisms may be, they do not qualify as flexible under our definition.
Bacterial cells also have internal clocks and oscillators that control their growth and enable them to adapt to changes in their environment. Again, while these are highly adaptive, there is no appearance of a new pattern here: all that varies over time is the values of the output and input variables.
I conclude that while cellular regulation in bacteria is built upon an impressive array of inter-locking complex processes, some of which exhibit memory, there is nothing that can properly be described as flexible behaviour, according to the definition given above.
3.4.2 Phenotypic plasticity in bacteria
The phenotypic plasticity of bacteria also illustrates their adaptiveness on an individual level. For instance, bacteria are able to switch their genes on and off in response to environmental changes. This ability to regulate gene expression serves a biological function: it allows bacteria to conserve their energy, in order to synthesise products that maximise their growth rate (Bridges, 2002). Because this behaviour serves a biological function, it is amenable to Dennett's intentional stance. Should we, then, interpret this apparent flexibility in bacteria as a mental calculation of the optimal course of action in changing surroundings?
A commonly cited case of phenotypic plasticity in bacteria is the way in which the lac operon is regulated in E. coli bacteria. An operon is a regulatory system found in bacteria, where genes that code for functionally related proteins are bunched together along a strand of DNA, enabling protein synthesis to be controlled in response to the needs of the cell. The lac operon allows E. coli to use lactose as an energy source, and break it up into its constituent sugars: galactose and glucose. An operon may exist in one of two regulatory states: ON or OFF. The lac operon is subject to positive and negative forms of gene regulation. The operon's default state is OFF, but the presence of lactose induces the genes to turn ON (negative regulation). However, if there is glucose in the environment, the lac operon is not expressed, as bacteria prefer glucose to lactose as a source of food (positive regulation).
Impressive as this behaviour is, there are two reasons for not characteristing it in mentalistic terms. First, the behaviour does not meet the criteria for "flexible behaviour" listed in the definition given above. The mechanism (i.e. the relevant program statements) governing the expression of the operon does not vary over time; only the environmental conditions do. Changing conditions correspond to changes in values of the input variables (chemical concentrations). Even in a fixed pattern (see definition above), these values may vary. In other words, the observed response - i.e. the value of the "output" variable - may vary over time, but the underlying pattern, which governs the response, stays the same. As Kilian and Muller (2001) and Beisecker (1999) have argued above, only those processes in which the pattern of responding to external stimuli can be altered over time should be regarded as candidates for mental processes. (See Conclusion F.3 above.)
Second, the behaviour is purely externally triggered rather than self-initiated: it does not vary in response to any internal states. For this reason, it can hardly be said to embody a "decision" on the part of bacterial "agents" regarding which energy source they should use. The regulation of the lac operon in bacteria can be better understood using a goal-centred intentional stance rather than an agent-centred stance.
Apart from the ability to turn regulatory genes (such as the lac operon) on and off, there are several other kinds of phenotypic plasticity in bacteria, including the production of diverse offspring with distinct phenotypes (bet hedging); the production of variable offspring through a high mutation rate (hypermutation), which promotes survival in fluctuating conditions; and localized elevated mutation rates (Ancel Myers and Bull, 2002).
Bet hedging can be defined as a form of between-individual, non-genetic variation whereby organisms produce diverse offspring as a means of coping with environmental change (Ancel Myers and Bull, 2002, p. 552). The diversity of phenotypes ensures that at least some of the organisms's progeny will survive in an uncertain environment where conditions vary. Hypermutation, by contrast, is a form of "between-individual, genetic variation" in which "the rapid production of variants enables survival in rapidly fluctuating conditions" (Ancel Myers and Bull, 2002, pp. 553-554). There are several mechanisms: a bacterium with a deficiency in the enzymes needed to repair its DNA can give rise to a strain of hyper-mutating bacteria, while other strains may result from randomly jumping transposable elements that disrupt genes. Bacteria may also exhibit higher mutation rates at particular locations, or hotspots, either as a result of random transitions between different ways of expressing a particular gene (phase shifting), or due to mutations at certain repeat sequences called microsatellites, which reduce the fidelity of the replication process.
However, bet hedging and hypermutation are even less promising candidates for intelligent behaviour by bacteria than the regulation of the lac operon. Ancel Myers and Bull explain why:
... whereas some forms of phenotypic plasticity involve appropriate phenotypic responses to environmental cues, bet hedging and hypermutation produced a range of variants without measuring the environment, only some of which will be appropriate for the current conditions. The SOS response of bacteria is a form of hypermutation that is both 'direct' and 'random' simultaneously. Certain environmental stimuli, including heat shock, radiation and chemical stress, cause the rate of spontaneous mutation to increase. The resulting variation arises in direct response to environmental stresses, yet the nature of that variation is often random, and often not appropriate for the environment (2002, p. 554, italics mine).
From the foregoing description, it should be clear that these adaptive forms of bacterial behaviour cannot be interpreted in mentalistic terms. First, they are not even amenable to the intentional stance: the environment is not measured, so no information about it is stored. The application of this stance requires, at the very least, a specification of the organism's information and goals. (See Conclusion I.1 above.)
Second, because the particular mutations that are triggered by environmental stimuli are un-directed (i.e. random) and usually inappropriate for their environment, they cannot be described as an intelligent response to changing circumstances.
Third, these forms of behaviour are not self-initiated: they are triggered by external environmental stimuli. It would be a misuse of language to describe these kinds of behaviour as manifestations of "agency" or "decision-making" on the part of bacteria.
Finally, the behavioural versatility displayed does not occur in an individual bacterium, but in a lineage of related bacteria. Even if we were to regard this adaptive behaviour as intelligent, then we would have to impute intelligence to an extended super-organism rather than to an individual bacterium. However, we have already argued (Conclusion B.4) that an evolutionary lineage cannot be meaningfully described as having mental states.
In short, while bet-hedging, hypermutation and localised elevated mutation rates certainly illustrate the impressive versatility of bacteria, the adaptibility they manifest does not qualify as truly flexible behaviour according to the definition given in Section 3 of Part B, and therefore cannot serve as evidence for mental states.
3.4.3 Gene-swapping in bacteria: flexible behaviour?
Gene swapping between individual bacteria is perhaps the most interesting kind of adaptive behaviour, because it informs other bacteria about what is going on, allowing them to adapt to unexpected environmental challenges like toxic mercury. In some ways, this phenomenon appears to be an even better candidate for true learning in bacteria than previous examples. Some bacteria have genes which make them resistant to mercury, as it is a naturally occurring toxin. The most widely studied and sophisticated mechanism for resistance to mercury works by bacteria exchanging transposons, or autonomous mobile sections of their DNA. Some transposons contain genes which confer resistance to mercury, by coding for specialised proteins and enzymes. Bacteria that have (or acquire) these genes can take highly toxic mercury ions into their cytoplasm (cell body) using their specialised carrier proteins, and transfer them to a specialised enzyme. The enzyme reduces ionic mercury to metallic mercury, which is relatively inert and non-toxic, and readily diffuses out of the cell (German Research Centre for Biotechnology, 2002; Petkewich, 2002).
Does the ability of bacteria to acquire genes that allow them to respond to challenges that they were previously unable to cope with, require a mentalistic explanation? At first glance, it may seem so: bacteria which pick up resistance to mercury are acquiring new information from other bacteria about an environmental hazard. Moreover, bacteria which acquire genes for resistance to mercury "can make new substances for new irreversible and reproducible information transfer paths as an answer to a new, formerly not identifiable stimulus" (Kilian and Muller, 2001, p. 3).
Certainly, there can be no doubt that the bacteria's changing response to mercury is an instance of truly flexible behaviour. The acquisition of new genes corresponds to a change in the program statement describing the organism's response to mercury, as well as the acquisition of new functions - i.e. new recipes for making specialised proteins and enzymes to handle the toxin. It appears that such gene swapping is a common occurrence among bacteria: for instance, genes which confer resistance to antibiotics can be passed from one species of bacteria to another (Society for General Microbiology, 2003). In fact, gene swapping appears to be a universal trait of organisms, as illustrated by the frequency of lateral gene transfer between different branches of the tree of life.
This leads us to the conclusion that all organisms in nature exhibit flexible behaviour, to some degree.
Case study: Does sensory adaptation in bacteria imply cognition on their part?
Whereas habituation can be defined as the waning in an organism's response to a stimulus because it is repeatedly presented to the organism, sensory adaptation (not to be confused with sensitization) refers to an even more primitive phenomenon, where the response may diminish because the organism's sensory organs adapt to the stimulus after an intense or prolonged period of stimulation and no longer detect it. Di Primio, Muller and Lengeler (2000) cite a case of sensory adaptation as evidence of learning in bacteria:
Interestingly, when a suspension of stimulated bacteria is rapidly mixed with a solution containing no ... attractant ..., they first increase tumbling ... From the observer's point of view the above finding could also be interpreted as if the bacteria were "surprised" about the disappearance of the attractant they were first following and the ... tumbling could be a sign of them "expecting" it (while "looking around") to reappear. After a while (we could say, because they "notice" that there is no advantage in doing so), they resume the normal unbiased run-tumble rhythm (2000, pp. 6 - 7).
However, the chemical basis of this adaptation is well understood. The presence of an attractant activates a chemical sensor which causes the cell's motor to "run" toward the attractant, while immediate removal of the attractant generates unco-ordinated motion or cell tumbling. In the meantime, a gradual "demethylation" process (which takes several minutes) switches the sensors and causes the cell to return to its usual run-tumble rhythm (Di Primio, Muller and Lengeler, 2000, p. 7).
The point I wish to make here is not that a chemical explanation precludes a mentalistic one. Rather, the point is that if we are to adopt a mentalistic account, it has to do some extra scientific explanatory "work" which a chemical account cannot do.
A further reason for rejecting a mentalistic explanation here is that the bacteria's behaviour conforms to a fixed pattern of behaviour, as defined in the previous section, and as such, does not warrant the ascription of cognitive mental states (Conclusion F.2). Roughly, the direction of the bacteria's motion is a function of the recent changes in the concentration of the attractant and the rate of demethylation of the sensors. To represent this mathematically, we need three variables: the current concentration of attractant; the last measurement, made 3 seconds ago (a time-lag variable); and the rate of demethylation.
The question of which organisms are capable of undergoing habituation and sensitization is important, because these processes are regarded by psychologists as a form of learning, defined broadly as a relatively permanent modification in an organism's behaviour as a result of experience (Abramson, 1994, p. 2). Some researchers have claimed that habituation occurs in all cellular organisms, even bacteria (Di Primio, Muller and Lengeler, 2000, p. 7). Are these organisms capable of genuine habituation (i.e. "learning" as defined by psychologists), or is there some other, simpler explanation?
Case study 1: Are bacteria capable of genuine habituation?
Abramson (1994) makes a distinction between habituation and sensory adaptation, which is one of several factors that can complicate habituation experiments. An organism's response to a stimulus may decrease because it adjusts to the repeated presentation of the stimulus. This is habituation, properly speaking. Alternatively, the response may diminish because the organism's sensory organs no longer detect the stimulus - a phenomenon known as sensory adaptation (not to be confused with sensitization).
Effector fatigue is another possible reason for the decline in an organism's response: the bodily mechanisms (effectors) that respond to stimulation may tire over the course of time.
Finally, some organismic responses diminish naturally over time, even without stimulation, so the (underlying) base rate of responding needs to be established before experiments are performed.
These distinctions are philosophically significant, regardless of whether one views habituation as a form of learning or not: ignoring an irrelevant stimulus is a very different thing from failing to detect it, or being too tired to respond to it, or simply slowing down over the course of time.
First, the ability to ignore a stimulus serves an important teleological function: screening out irrelevant stimuli. By contrast, natural slow-down, fatigue and failure to detect a stimulus serve no biological function.
Second, while ignoring a stimulus could be considered as a self-initiated activity, sensor failure, fatigue and natural slow-down can hardly be said to qualify as activities of any kind.
Di Primio, Muller and Lengeler (2000, p. 7) claim that "[w]ith bacteria, it is impossible to draw a dividing line between habituation and [sensory] adaptation", and the Encyclopedia Britannica article (1989, Vol. 1, p. 872) agrees that "these distinctions make rather little sense in the case of a single-celled animal." However, according to Abramson (1994, pp. 108 - 109) there are two commonly used procedures for differentiating between habituation and sensory adaptation: make the time between two successive presentations of the habituation stimulus long enough for adaptation to wear off, or perform follow-up testing of the effects of habituation, some time after the organism has been trained to diminish its response.
Effector fatigue can be distinguished from habituation in the same ways, or by presenting the organism with a second stimulus from a different sensory modality (e.g. vibration instead of light) to see if the same response (e.g. withdrawal) can be elicited. If it can, then fatigue can be ruled out.
There is no reason why these procedures could not be applied to bacteria.
The evidence cited by Di Primio, Muller and Lengeler (2000, p. 7) for habituation in bacteria is the observation that following prolonged exposure to an attractant, they change from a "run" to a "tumble" movement. However, if we examine the chemical basis for so-called "habituation" in bacteria, it appears to be a case of sensory adaptation, rather than habituation. As Illingworth puts it:
With increasing attractant concentrations the MCPs are progressively converted into the fully methylated state with a low affinity for the attractants (1999).
In other words, at high concentrations, bacterial receptors become less sensitive. This effect wears off when methylation of bacterial sensors is reversed (by removal or dilution of the attractant). If we follow Abramson's suggestion that time trials can be used to distinguish habituation from sensory adaptation, then the hypothesis that the observed waning of responsiveness in bacteria is explicable in terms of sensory adaptation entails that the removal of a stimulus (e.g. an attractant), followed by its presentation after an interval of time, should cause a bacterium to respond in the same way as it usually does when exposed to an attractant. As far as I have been able to ascertain, this is indeed the case. (If the decrease in response were due to habituation instead, it should persist even when the attractant is removed, thereby de-methylating the bacterium's sensors.)
Case study 2: Habituation in protoctista

Certain kinds of protoctista (especially protozoa, such as paramecia and amoebae) are recognised as being capable of being properly habituated (Abramson, 1994, pp. 106, 112, 116, 117). This is an adaptive feat which bacteria appear to be incapable of.
Wood (1988, 2003) describes how the protozoan Stentor can be habituated to mechanical stimulation; the presence of intra-cellular calcium ions is critical to the process. Before habituation can be identified in an organism, alternative explanations have to be ruled out - especially sensory adaptation and effector fatigue, which were discussed in the section on bacteria above. Wood explains why he believes the behavioural change observed in Stentor is a genuine case of habituation:
Briefly, it is not sensory adaptation since repetitive stimulation of the sensory surface does not produce a decrement in the amplitude of the recorded receptor potential...Likewise, it is not motor fatigue, since the threshold for contracting to electrical stimuli is not altered by habituation by mechanical stimuli... (personal email, 18 June 2003).
Wood also argues that habituation in protozoa is biochemically distinct from adaptation in bacteria, which was rejected earlier as a possible instance of habituation:
Based on the criterion of similar cellular and biochemical processing, it is clear that adaptation in bacteria, which involves only chemical processes and not ion channels is a rather different phenomenon than habituation in protozoa (personal email, 18 June 2003).
Case study 3: Habituation in plants
According to Abramson, Garrado, Lawson, Browne and Thomas (2002, pp. 174-176), studies of behaviour modification in plants go back to 1873. Most studies of plant learning have examined habituation in Mimosa, a small shrub whose leaves are sensitive to stimulation. Mimosa leaflets close in response to touch, heat, electrical shock or puffs of air. In the absence of further stimulation, the leaflets re-open after about 15 minutes. Exposure to stimuli also makes the stem fall downward, but after the same interval of time, the stem rises again. In 1873, the researcher Pfeffer induced habituation in Mimosa leaflets: after repeated stimulation, the leaf-closing behaviour in response to stimulation was no longer observed. In 1906, another scientist, Bose, found that constant electrical or mechanical stimulation of the stem initially triggered a falling response, followed by a slow rise, after which the response could be triggered again. However, repeated stimulation led to a loss of response to the stimulus. Follow-up research in 1972 showed that increasing either the interval between trials or the intensity of the stimulus also increased the time required for habituation.
Habituation is also known tp occur in other plants: the carnivorous plant Drosera, known as the Sundew, and the Passion Flower Passionflora gracilis (Abramson, Garrado, Lawson, Browne and Thomas (2002, p. 175).
Aside from non-associative forms of habituation, there is experimental evidence that that more complex forms of habituation may be found in Mimosa. An experiment by Holmes and Gruenberg in 1965 showed that Mimosa could discriminate between different types of stimuli: it could be "trained" to stop closing in response to water droplets, but still retained its response to the touch of a finger, indicating that the change in response was not due to fatigue.
One could conclude from the results of the experiment that the plant had learnt to do something new: discriminate between two stimuli. This would imply that the habituation observed here was associative rather than non-associative in nature. Alternatively, there could be different response mechanisms within the plant for stimuli of different weights. The case remains intriguing but inconclusive. Clearly, more research needs to be done here.
Case study 1: Are protoctista capable of associative learning?

It has been claimed that paramecia possess a capacity for learning through classical conditioning. The original experiment was reported in a study by Hennessey, Rucker and McDiarmid (1979) and is still widely quoted (e.g. by Martin and Gordon, 2001). However, as far as I have been able to ascertain, no-one has replicated, or even attempted to replicate, this result. In accordance with the conservative methodological principles laid down earlier, I shall not consider this evidence. For the time being, it would be unwise to ascribe associative learning to paramecia.
Hinkle and Wood (1994) dispute the existence of associative learning in protozoa. In their study, they examined a claim that the protozoan Stentor exhibited a form of instrumental conditioning while learning to escape from a capillary tube over repeated trials. In a recent personal email communication, Wood (personal email, 18 June 2003) writes:
Our results indicated this phenomenon is not a real example of instrumental conditioning (Hinkle and Wood, 1994).I also know of no other well substantiated cases of associative conditioning in ciliates.
It should be noted that the experiment reported by Hennessey, Rucker and McDiarmid (1979) was conducted on Paramecium (a quite different type of ciliate). Clearly, further investigation is warranted.
Case study 2: Are plants capable of associative learning?
Research into the possibility of classical conditioning in Mimosa has produced negative or conflicting results, and the methodology of studies which found conditioning has been criticised (ibid., pp. 175-176). Attempts to condition Mimosa with light touch as the conditioned stimulus (CS) and electrical or mechanical shock as the unconditioned stimulus (US) failed. Other attempts, using light as the CS and touch as the US yielded positive results in two (arguably flawed) studies, and negative results in another study. In keeping with my methodological constraints, these studies will be ignored here.

Abramson, Garrado, Lawson, Browne and Thomas (2002, pp. 173 - 185) describe an experiment of their own, designed to verify the existence of associative learning in plants, using a newly refined method. Different groups of Philodendron cordatum plants were exposed to a six-hour training period of light only, dark only, or alternating one-minute periods of light and dark. Following training, all plants were exposed to a ten-minute testing period in darkness, when their bioelectrical potentials were recorded, using EEG equipment. As expected, the different groups showed a difference in amplitudes, reflecting differences in their prior exposure to light: the plants were using some physiological mechanism to store information about their history of exposure (i.e. some kind of memory). Such an explanation is compatible with a non-mentalistic account - the plants could have been just retrieving stored information.
However, if learning had occurred, the researchers reasoned, they would expect to see differences in the amplitudes for the group exposed to one-minute intervals of light and darkness, between minutes 1, 3, 5, 7 and 9 on the one hand, and minutes 2, 4, 6, 8 and 10 on the other, corresponding to the switching of the light in the training period. The researchers therefore looked for differences corresponding to the switching of the light in the training period. No such differences were found, although the group cautioned that one-minute intervals may have been too short to allow the plant to adjust its responses to changes in the light. The authors concluded:
At this point we cannot conclude it is possible for learning to occur in the absence of a nervous system. Further studies should examine the effect of longer intervals of light and dark exposure (2002, p. 184).
Another reason for caution regarding the above experiment is that plants may simply be capable of learning, but poor at timing. Temporal conditioning, where a single stimulus is presented at regular intervals that the subject learns to anticipate, has been demonstrated in vertebrate animals, but to date, attempts to demonstrate this phenomenon in invertebrates, including honey bees, crabs, earthworms and flatworms have failed (Abramson, personal email, 2 February 2003). Is it reasonable to expect plants to be able to do what most animals cannot?
Case study 3: Are worms capable of associative learning?
The relationships between the different groups (or phyla) of worms, and other animals, is shown below. All major groups (phyla) of animals, apart from "simple" animals such as sponges and cnidaria, have three tissue layers and are bilaterally symmetrical, with upper-lower and left-right axes. Additionally, they have a distinctive neurophysiological property: a "central nervous system, organised around a massed concentration of nerve cells called the cephalic ganglion - the archaic brain" (Prescott, 2001). All phyla of worms possess this feature.
Bilaterally symmetrical animals fall into two groups: protostomes (which have one opening that serves as both a mouth and an anus) and deuterostomes (which have a mouth and an anus). Protostomes are by far the bigger group, and are made up of two sub-groups: ecdysozoans, or moulting animals, and lophotrocozoans.
The bodies of ecdysozoans are covered with an outer layer of light, thin, organic material (which some of them shed periodically) that functions as a skeleton. Unsegmented worms (e.g. nematode roundworms, such as the well-studied Caenorhabditis elegans, and horsehair worms) and arthropods (insects, spiders and crustaceans) belong to this large group.
Most other phyla of worms, including segmented worms or annelids (e.g. earthworms) and flatworms (or platyhelminthes), as well as molluscs and lamp shells belong to the group known as the lophotrocozoans, a seemingly disparate clade of animals whose embryos develop in a similar fashion.
Only vertebrates, their close relatives and echinoderms are classified as deuterostomes. "Of the deuterostomes ... vertebrates are the only animals with highly developed nervous systems (Prescott, 2001, p. 4, italics mine).
Flatworms (planaria) are believed to be the most "primitive" group of worms.
Case study 3(a): Some procedural pitfalls in verifying conditioning in animals
Pseudo-conditioning
One of the main pitfalls researchers need to guard against, when attempting to identify conditioning in animals, is pseudo-conditioning. Pseudo-conditioning refers to behaviour mimicking conditioning, whereby "presentation of the US [unconditioned stimulus - V.T.] can affect the animal so that a subsequently presented CS [conditioned stimulus - V.T.] produces a 'CR' [conditioned response - V. T.], even though the CS and US have never been paired" (Abramson, p. 156). Likewise, in instrumental and operant conditioning trials, the reinforcement alone may produce a behavioural change resembling the change which is supposed to be produced by conditioning. Abramson gives an example of a crab which is trained to press a lever for food, by being rewarded with squid after each bar press. After the first few bar presses, the rate of pressing increases dramatically. Is this because the crab has associated the lever with the reward (a mentalistic interpretation) or because of the energizing effect of the food, independent of any link between response and reward? According to Abramson (1994, pp. 129, 156), pseudo-conditioning can be excluded by training the animal to discriminate between two stimuli, where only one is paired with the US (in classical conditioning experiments) or where only one leads to the reward (in the case of instrumental conditioning). For instance, if the crab in the above experiment were able to discriminate between two levers, only one of which is reinforced by food, then conditioning would be demonstrated.
Sensitization
Another explanation for apparent "conditioning" in animals is sensitization. What is supposed to happen in classical conditioning is that the animal learns to associate a neutral event (the conditioned stimulus) with a biologically significant non-neutral event (the unconditioned stimulus). However, Abramson cautions that often, researchers working with invertebrates end up using a conditioned stimulus which is non-neutral, as it "already elicits the conditioned response, albeit in a reduced form" (personal e-mail, 2 February 2003). This is a pervasive problem with studies of invertebrate learning:
Unfortunately, most of the CSs used in invertebrate behavioral experiments elicit the response that is to serve as the index of learning (Abramson, 1994, p. 133).
If the index of "learning" is the same as one of the responses elicited by the CS prior to pairing, then measurement of learning becomes problematic. Abramson discusses this issue in connection with planarians (a kind of flatworm):
The planarian work revolved around the same issues. It is difficult to find a CS that does not produce a response resembling a conditioned response prior to pairing (there is only so much variety in the behaviour of worms) (personal email, 2 February 2003).
Alpha conditioning
Some researchers have attempted to circumvent this problem by habituating the animal to the CS before conditioning trials begin, in order to make it seem benign or "neutral" to the animal. When the animal ceases to respond to the CS, the CS is then paired with the US, until the animal responds to the CS in the same way that it (innately) responds to the US. The problem with this procedure is that the new response elicited by the CS may simply be due to a re-awakening of the animal's old, innate response to the CS (by pairing it with the US, which evokes a similar response), rather than a learned association between the CS and the US. On the former explanation, the animal's behaviour can be explained as a kind of sensitization known in the literature as conditioned sensitization. Other authorities refer to it as alpha conditioning or US-US conditioning, as the CS elicits an innate response and should therefore be described as a (non-neutral) US rather than as a (neutral) CS (Abramson, 1994, p. 106). The difference between the two explanations is that in the former case, the animal is not learning to do something new; it is merely being habituated to a stimulus and then re-sensitized to it. As Abramson puts it:
If we go back to the planarian work, standard procedure was to first HABITUATE responses to the CS prior to pairing with the US. In my view this experimental design is not classical conditioning... After the first presentation of the US the animal is now sensitized once again... If you were to plot the performance (conditioned response) of such a procedure, the first CS trial begins at '0' and the reader has the impression that the CS was neutral (personal e-mail, 2 February 2003).
Other factors
Other factors that may lead to false positive results in conditioning trials are: (i) the base rate of responding (where the animal shows a tendency to respond spontaneously, without any stimulation); (ii) the animal's central excitatory state or CES (where its nervous system may be excited by the presentation of the unconditioned stimulus, so that it responds to stimuli which it would not normally respond to - including the conditioned stimulus); (iii) pheromones, or signalling odors, which may contaminate the experimental apparatus and alter the animal's behaviour on subsequent trials); (iv) calendar variables (e.g. seasonal or local weather changes) which affect animals' behaviour; and (v) experimenter bias (e.g. subtle differences between researchers in the way they present stimuli to the animal) (Abramson, 1994, pp. 130-132, 157-159). Abramson points out that contamination of results by these factors can be avoided by using suitable control groups, longer inter-trial intervals, proper cleaning procedures and "blind" experiments (to rule out bias). However, the problem is that conditioning trials for invertebrates are seldom conducted rigorously enough to rule out these factors.
Pitfalls in experiments with flatworms
Claims by scientists to have identified classical conditioning in planaria (flatworms) have been discredited by subsequent research. The following account illustrates how sloppy experimental controls can lead researchers to draw premature conclusions about animals' capacities for classical conditioning:
Classical conditioning of planaria was first reported by R. Thompson and J. V. McConnell in 1955. The planaria became a fairly common model for studying conditioning and memory in the 1960's. In 1967, Richard Block and McConnell published an article in Nature, that reported results of classical conditioning in brown planaria (Dugesia dorotocephala). In their study, they paired an electrical shock ... with a flash of light ... When the shock would occur, the worms would contract and turn at the anterior end. The experiment was controlled by a group that received no electric shock, and by a group that received light and shock at random. After training, the planaria were given stimulus by light alone without electric shock. The planaria would exhibit the same type of response that was originally caused by the electric shock. Block and McConnell followed this with extinction trials, that is they "reverse trained" the planaria to forget their earlier learning.Studies of this kind fell out of favor in the early 1970's, as some researchers, notably Allan Jacobson, Sheldon Horowitz, Clifford Fried, John Hullett, and M.J. Homzie, argued that the training was not true classical conditioning, but rather that the planaria exhibited "pseudo-conditioning" and "sensitization" (Duane, Fortenberry and Horowitz, 1998).
Pitfalls in experiments with earthworms
In a similar vein, Abramson and Buckbee (1995) report on four experiments they conducted with earthworms (Lumbricus terrestris). Earthworms represent what Abramson (1994, p. 177) calls "the next evolutionary advance" beyond flatworms, in that their body is divided into similar segments, which "can respond individually or as a group, and can be modified to perform special tasks" (1994, pp. 177-178). By using multiple control procedures, Abramson and Buckbee demonstrate that much of what has been described as "associative learning" in earthworms is actually non-associative in nature. Earthworms were trained to respond to the scent of rose (the conditioned stimulus) in the same way as they responded to the smell of n-butanol (the unconditioned stimulus, to which earthworms have an innate aversive response). The fact that earthworms who were exposed to the US before being exposed to the CS (backward conditioning) responded no differently from earthworms who were exposed to the CS before the US, tells against the hypothesis that they had learned to associate the CS with the US, and in favour of the hypothesis that their response was due to sensitization. Previous experiments have tended to overlook backward conditioning; instead the control group used has been one where the CS and US are simply not paired. Abramson and Buckbee suggest that "relying solely on an unpaired group may underestimate the amount of pseudo-conditioning" (1995, p. 394). They also note that they "were surprised to find no studies in the worm literature directly measuring the extent of pseudo-conditioning" (1995, p. 394).
Abramson and Buckbee (1995, p. 395) also describe an experiment in which they attempted to measure the extent to which earthworms' responses to the scent of the CS were caused not by an association between the CS and the US, but by a carry-over excitation of their nervous systems arising from the presentation of the US during a previous trial, making them respond to stimuli which they would not normally respond to - including the CS. What passes for a conditioned response may in fact be due to the central excitatory state (CES) of the subjects. In their experiment, Abramson and Buckbee conditioned the worms in 20 trials where the CS was immediately followed by the US, and then (one minute later) by a third stimulus that was supposed to dissipate the lingering effects of the US, before the next trial. Trials were spaced at 2-minute intervals. The worms were equally likely to respond to the third stimulus (which did not predict the presence of the US) as to the CS, suggesting that the third stimulus "did not effectively discharge unconditioned stimulus excitation or contained carry-over excitation of its own" (1995, p. 395).
Summing up, the authors conclude that the animals failed to display true classical conditioning, and that their apparent "learning" responses "can be interpreted as an example of sensitization as measured by either a pseudo-conditioning or by a CES design" (1995, p. 395). They suggest that "sensitization appears to be the underlying behavioural mechanism of what has been previously characterized in the earthworm as classical conditioning phenomena" (1995, p. 396).
An over-drawn contrast?
Finally, the contrast which is often drawn between two forms of "learning" - non-associative (where the animal modifies its response to one kind of event but does not learn to do anything new) and associative (where the animal learns to associate two kinds of events) - may be an over-simplification. Work by Rose and Rankin (2001) suggests that associative cues can facilitate habituation: C. elegans worms remember their habituation better if they are tested in the same environment they were trained in.
Summary
The main moral we can draw from this is not that worms are incapable of associative learning, but that rigorous experiments need to be performed to demonstrate exactly what kinds of learning they are capable of. As I show below, there are in fact some well-conducted experiments which establish that most if not all worms are capable of undergoing associative learning, in the proper sense of the word.
Case study 3(b): The current state of evidence for associative learning in worms
So far, the evidence presented for associative learning, even in worms with more complex nervous systems, appears to be negative. However, recent research with another worm, the well-studied nematode worm Caenorhabditis elegans, indicates that some worms are after all, capable of associative learning. C. elegans belongs to the phylum Nematoda (roundworms) and is a favourite of scientists studying the genetic and molecular bases of learning, because it has a very simple, fully mapped nervous system (302 neurons) and a small, almost completely sequenced genome. Although they are protostomes (animals with one opening that serves as both a mouth and an anus), roundworms are not closely related to flatworms; they belong to a separate clade known as Ecdysozoans (moulting animals), along with arthropods (insects, spiders and crustaceans) and a few other phyla.
Conditioning in C. elegans is distinguishable from conditioned sensitization (alpha conditioning)
Experiments suggest that the underlying mechanism for conditioning in C. elegans - even so-called "alpha" conditioning - is different from that of non-associative learning (simple habituation and sensitization). In particular, some mutant worms have been found to be normal in tests of non-associative learning, but utterly incapable of short-term or long-term associative conditioning, unlike other, normal specimens (Morrison, Kumar, Wen, Runciman, Neghandi and van der Kooy, 1995). In the experiment which prompted this finding, C. elegans worms were conditioned to prefer one attractive chemical stimulus (Na+ ions) over another (Cl- ions) which is equally preferred under normal conditions, simply by being exposed to the former in the presence of, and the latter in the absence of, a food source (E. coli bacteria). What we have here is a case of US-US conditioning, where E.coli is the unconditioned stimulus (US) and Na+ ions represent the so-called conditioning stimulus (CS), which can also be viewed as an unconditioned stimulus, because it is attractive to C. elegans.
It was also shown that two lines of mutant worms (lrn-1 and lrn-2) do not alter their preferences after this kind of conditioning. This research appears to demonstrate that associative and non-associative learning are not only behaviourally distinct, but also genetically distinct. If this is correct, then it is inaccurate of Abramson to characterise alpha-conditioning (or US-US conditioning) as conditioned sensitization (a non-associative form of learning).
Radical changes of preferences in C. elegans
Another study (Morrison, Wen, Runciman, van der Kooy, 1999) shows that C. elegans worms can actually be conditioned to avoid a previously attractive stimulus - in other words, radically alter their preferences. Once again, lrn-1 and lrn-2 mutants show no change:
In a new olfactory associative learning paradigm, in which wild type worms learn to avoid a previously attractive diacetyl odor after it has been paired with an aversive acetic acid solution, lrn-1 and lrn-2 are impaired. Although defective in associative learning using a conditioned olfactory cue, nonassociative learning (habituation and dishabituation) using this same olfactory cue is unaffected.
This change of preference cannot be explained away as "conditioned sensitization" because the old response is not re-awakened. The worms are actually learning to do something new: they are changing their pattern of response to a stimulus. The fact that nematode worms can be conditioned in this way indicates that they are capable of a kind of behavioural flexibility which bacteria, protoctista, plants and the simplest animals do not exhibit. According to Kilian and Muller's definition, this surely qulifies as "true" learning.
Saeki, Yamamoto and Iino (2001) report similar results, in which C. elegans worms starved for several hours on a plate containing salt (NaCl) learn to avoid the salt, which is otherwise attractive to worms. The authors write:
This conditioning requires both the presence of NaCl and the absence of a bacterial food source, indicating that it is not merely adaptation or habituation, but that it is likely to be a form of associative learning (2001, p. 1757).
Summarising recent research, Rankin (personal email, 31 May 2003), whose specialty is learning in C. elegans, writes:
I disagree with Abramson - I believe that many invertebrates are capable of classical conditioning. Work on C. elegans with chemical cues (van der Kooy, and our context work) and thermal cues (Mori) show C. elegans can make associations between a reliable CS and a US.
Abramson's cautionary remarks about the difficulties of identifying associative learning are well-taken; nevertheless, behavioural and neurological research in the last few years suggests strongly that even worms like C. elegans, with simple nervous systems, are capable of classical conditioning.
Case study 3(c): Are worms capable of undergoing instrumental conditioning?

Instrumental conditioning or sensitization?
Even the "simplest" flatworms (planaria) are capable of learning to respond more quickly to an aversive stimulus, as described by Abramson et al.:
For instrumental conditioning the turning response consists of a contraction followed by an extension of the animal. Following several contractions and extensions the animal begins to turn away from the direction of the airpuff and swims in the opposite direction. For example, if the animal is swimming in a clockwise direction repeated presentations of an airpuff elicits a number of contractions and extensions. These behaviors are followed by the animal turning and swimming in a counterclockwise direction. In our demonstration the dependent variable of interest is the number of airpuffs required to make the animal swim in the opposite direction. As training progresses the number of airpuffs required to produce a turning response steadily declines (Abramson, Kirkpatrick, Bollinger, Odde and Lambert, 2001).
However, these observations could be interpreted in various ways. Is the animal learning to associate its behaviour (swimming direction) with a punishment (the air puff) - i.e. undergoing instrumental conditioning - or is it simply becoming sensitized to the air puff (non-associative learning, as distinct from "true" learning)? Is the animal really "trying out" different directions of swimming, or is it simply avoiding an aversive stimulus to which it has become sensitized?
Instrumental conditioning or classical conditioning?
Classical conditioning may also be confused with instrumental or operant conditioning. Even the study which showed that C. elegans worms could be conditioned to alter their food preferences, can be interpreted as operant conditioning, as the study's author acknowledged (Nuttley, personal email, 27 August 2003):
I've had people comment that what I am looking at is actually operant, and they make a good point. In my PNAS paper I show that the animals either approach or avoid benzaldehyde depending on whether it was previously paired with food (reward) or starvation (punishment). On the test, it is their chemotactic behaviour in response to the (trained) CS that is scored. Although the behaviour does not produce reward (food), we assume that they are acting appropriately to that 'expectation'.
Case study 3(d) Are worms capable of undergoing operant conditioning?
It is generally acknowledged that most worms, with the possible exception of flatworms, are capable of undergoing instrumental conditioning, if this is defined as "behaviour controlled by its consequences" (Abramson, 1994, p. 151).
Many behavioural scientists use the terms "instrumental conditioning" and "operant conditioning" interchangeably, but others (e.g. Abramson, 1994) distinguish the two, defining the latter more narrowly as behaviour in which "an animal must demonstrate the ability to operate some device - and know how to use it, that is, make an arbitrary response to obtain reinforcement" (1994, p. 151). In a recent email, Abramson added:
I would be more convinced that an invertebrate has operant responses if they can adjust their, for example, swimming speed to fit the contingencies. These studies have not been performed (personal email, 2 February 2003, italics mine).
C. elegans is capable of undergoing instrumental conditioning, in the broad sense defined above. Recently, I emailed Catharine Rankin (an acknowledged specialist in learning processes in C. elegans) and asked her about Abramson's restrictive definition of operant conditioning. In response to my query "Do we know if C. elegans is capable of true operant conditioning?", Rankin replied, "No-one has shown this" (personal email, 31 May 2003).
However, there are some very recent studies that suggest that C. elegans may be capable of operant behaviour, after all. It is well-known that C. elegans migrates toward a preferred temperature on a thermal gradient. Zariwala, Miller, Faumont, and Lockery (2003) subjected unrestrained wild-type worms and mutant cryophilic (cold-loving) and thermophilic (heat-loving) worms to sudden temperature "steps" of 3??C up or down from the temperature in which they had been raised (20??C). An "ethogram" was constructed, and the worms' principal ways of moving (or "behavioural states") were identified - forward locomotion, "reversals" (in which a worm moves backward for several seconds and then goes forward again in a new direction), and "omega turns", (where the worm's head bends around to touch its tail during forward locomotion, momentarily forming a shape like the Greek letter). Statistical analysis had previously shown that reversals and omega turns occur in bursts that have been termed "pirouettes". The researchers tested the hypothesis that worms tend to pirouette more when they are moving away from their preferred temperature (as pirouettes might serve to direct worms back to this temperature), while their tendency to turn should decrease when they are moving toward their preferred temperature. The authors of the study summarised their research as follows:
Overall, turning probability was modulated in a manner consistent with a role for turns in thermal migration, although not always as predicted by the pirouette hypothesis in the strict sense (2003, p. 4369).
The authors cautioned that theirs was only the second study of its kind (relating to thermotaxis in C. elegans), and that the previous study (performed in 2002) had produced somewhat different results, possibly because of "significant differences between the temperature stimulus and the definitions of turning behavior used in the two studies" (2003, p. 4376).
Additionally, Nuttley (personal email, 27 August 2003) cites research demonstrating that C. elegans worms are capable of navigating past some aversive barriers of high osmolarity in order to reach an attractive odour. This might indicate some degree of control.
Clearly it would be premature to draw any conclusions from this research, but at least it shows how one might proceed in attempting to verify operant behaviour (in Abramson's sense) in worms. First, it is important to catalogue the patterns of bodily movements of which the test animal is capable (i.e. define an "ethogram"). Second, it is essential to know what attracts it and what repels it. Third, the animal has to control its bodily movements in order to obtain the "reward" or avoid the "punishment".
The real question is: what counts as "control over bodily movements"? An increase in a worm's tendency to turn when it becomes too hot or cold might be a case of controlled behaviour, but it could also be an aversive reaction to change. However, if a worm were able to adjust, say, the rate or degree of its movement as temperature varied, this would be a more clearcut case of adjusting a response to fit the contingencies, as Abramson demands.
Pavlov proposed his stimulus substitution theory to explain the conditioning process: all that happens is that the unconditioned stimulus (US) is substituted for the conditioned stimulus (CS), in eliciting a response. The conditioned response (CR) and unconditioned response (UR) are exactly the same. The animal does not actually learn anything; a new neurological connection is formed in its brain, and nothing more. Subsequent research has exposed the inadequacies of this view:
[I]f simple stimulus substitution were to account for learning, the CR should be identical to the UR. Several observations seem to indicate that this is not the case. Rabbits for instance, respond with swallowing and jaw movements during training in a salivary conditioning paradigm, but fail to show these behaviors during test... Or the CR might include behaviors not present in the UR: Pavlov's above-mentioned dog that showed appetitive behavior towards the bell is one example of motor activity towards CSs paired with food, although activity is not part of the response to food itself. Pigeons peck visual signals for USs that do not elicit pecking...Moreover, it has been shown that conditioning still does occur if the stimulus-properties of the US are suppressed... Suppression of the response-evoking properties of the US, for instance by applying response attenuating drugs such as curare does not prohibit (sic) learning either ... ruling out direct stimulus-response associations in classical conditioning (Brembs, 1996).
Brembs' biological account of associative learning (1996) is more representative of current thinking: an animal that is being conditioned is learning to re-evaluate stimuli, in relation to its own built-in needs:
Assume an animal struggling for survival: every sensation might provide a clue how to escape a predator, find a mate, explore new food patches, hiding places, etc. In every second it is confronted with potentially dangerous or advantageous situations. The possibility to predict such situations must convey an enormous selection pressure. A very effective way to accomplish this task would be an evaluation mechanism, judging situations according to their 'beneficence' for the individual. With such a mechanism salient internal and external stimulus-arrays extracted from the situation would receive situation-specific rankings on a value-scale in terms of 'good', 'bad' or 'neutral' (1996, p. 22).
Brembs claims that this account is compatible with several current models of conditioning, and can also account for various phenomena - including differences between the CR and UR - that have been observed by researchers who study conditioning. But if animals that are being conditioned are judging situations and learning to do something new (e.g. evaluate new stimuli or re-evaluate existing stimuli), then it is at least legitimate to raise the question of whether they possess cognitive mental states. However, Brembs himself prefers to avoid the word "cognitive", regarding it as scientifically unproductive.
Case study 1: conditioning in the autonomic nervous system of an astronaut in zero-gravity

Astronaut.
Ryder and Martin (1998) have attempted to discredit Dretske's account of belief by offering a counter-example. They have pointed out that the human autonomic nervous system (ANS) is capable of associative learning, yet few people would ascribe beliefs to it. Contrary to popular belief, the ANS does not simply follow fixed action patterns, but can be conditioned. The ANS is controlled by the central autonomic network (CAN), which is located in the brainstem, mid-brain and fore-brain. Each individual's CAN contains an indicator which represents whether she is standing, and which compensates for the loss of blood to the brain (caused by gravity) when she stands up, by perfusing her brain with blood. When an astronaut stands up in zero-gravity, her blood rushes to her head, because the ANS is used to counteracting the effects of gravity on earth. However, with time, the astronaut's ANS un-learns this compensating behaviour. When the astronaut returns to earth, her ANS has to re-learn the skill of compensating for the loss of blood to the brain when she stands up. The authors argue that the above example meets Dretske's criteria for genuine learning, and that according to his criteria, the ANS is capable of having beliefs (e.g. about the astronaut's posture) and desires (e.g. to adequately perfuse her brain with blood). On the other hand, the attribution of beliefs to the ANS sounds peculiar, so Ryder and Martin argue that Dretske's criteria for having a belief must be insufficient.
I am not sure that this counter-example decisively refutes Dretske, as an alternative explanation appears possible. In associative learning, an individual has to form an association between two events. However, one could try to explain the behaviour of the astronaut's ANS as a case of non-associative learning: in the absence of gravity, the ANS's compensatory response to standing naturally attenuates, but when the astronaut returns to earth, her body is re-sensitized, and the original response re-appears. Ryder has since acknowledged this point, but considers conditioning a more likely explanation for the autonomic behaviour observed:
The key is whether the response in microgravity is just a "relaxing" of the normal response, or if something more active is going on. As I recall, there was some evidence for the latter - for one thing, it seems to be linked to the discriminative stimulus, the vestibular inputs (personal email, 4 September 2003).
Case study 2: conditioned leg withdrawal in headless cockroaches

Cockroach. Clip art licensed from the Clip Art Gallery on DiscoverySchool.com
A more clearcut case of associative learning without a brain is leg withdrawal, which can be conditioned in headless cockroaches or in isolated leg and thoracic ganglion preparations. The thoracic ganglion is a much more complicated cluster of nerves in the cockroach than the brain (Kentridge, 1995). Cockroaches are thus capable of "distributed" learning. Yet it would seem strange to describe their leg withdrawal behaviour as a manifestation of a belief.
Case study 3: conditioning in the severed spinal cords of rats
Diagram of a laboratory rat, showing its spinal cord.
New research shows that severed spinal cords in rats are capable of undergoing associative learning.
Image courtesy of National Institute of Health, USA.
Another troubling case for Dretske is discussed by Grau (2002). Rats whose spinal cords had been severed at the second thoracic vertebra (T2), leaving them paralysed below their mid-sections, were shown to be capable of undergoing instrumental conditioning within their spinal cords. The rats were placed in an apparatus where a shock was applied to one of their hind legs, whenever it made contact a solution of salt water beneath the rats. The rats soon learned to maintain the leg in a flexed (up) position, thereby avoiding shock. Moreover, the duration of each flexion increased as the training continued, peaking at a little under a minute. Although the rats' hind legs were not controlled by their brains, their spinal cords were capable of being conditioned. The experiment was conducted with yoked control subjects (to exclude the possibility that the shock itself was causing the rats' hind legs to flex), and the response was also observed to occur later under normal conditions, verifying that learning had indeed taken place in the animals' spinal cords. While this case demonstrates that true learning can take place within an animal's nervous system in the absence of a brain, we would not normally explain this by saying that the rats' spinal cords acquired new beliefs. It would be more appropriate to say that they acquired a new response pattern.
These three examples undermine the notion that flexible behaviour patterns - even those that are generated by an internal mechanism (Conclusion F.6) - can serve to distinguish organisms with beliefs from those that lack them.
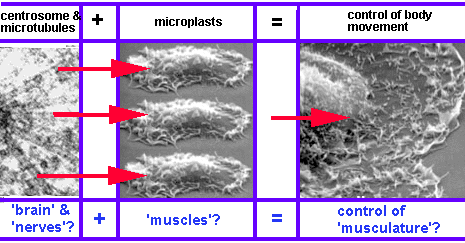
Case study: The lysis-lysogeny Decision in viruses

Image of influenza virus. Copyright Linda M. Stannard, Department of Medical Microbiology, University of Cape Town, 1995.
A well-known case of phenotypic plasticity in viruses is the lysis-lysogeny decision, whereby parasitic lambda-phage viruses adopt a bet-hedging strategy in order to cope with fluctuations in the availability of their hosts (E. coli bacteria). Preuss (2000) describes the process thus:
When a bacteriophage ("bacteria eater") virus injects its own dna (sic) into a microorganism such as Escherichia coli, the host cell apparatus rapidly expresses the program on the viral dna (sic) that decides whether or not to kill the host immediately. Under conditions that are less than optimal for replication, the phage may actually confer immunity to further infection upon the host (lysogeny). But if conditions are good, the virus produces so many copies of itself that the cell walls burst - a state known as lysis - and the infection spreads.Two independently produced regulatory proteins compete to control whether the invading genes will remain quiescent or be expressed. Because of inescapable thermal noise, the outcome in any given case is random, and the proportion of the population in either state changes according to conditions such as cell nutrition and the number of invading particles per cell...
Arkin and his colleagues have found that the underlying stochastic [i.e. random - V.J.T] mechanisms of the lysis-lysogeny decision circuit... depend entirely upon the chance timing and concentrations of bursts of competing proteins that act to reinforce or inhibit one another.
"...Thermal fluctuation at the molecular level makes for diversity in cells that start out under identical conditions," says Arkin. "The phage actually makes use of noise as a survival mechanism: sometimes it pays to multiply and infect as many hosts as possible, sometimes it pays to lie low. Either way, the viral population is prepared to cope with changing conditions" (italics mine).
Case study 1: The mechanism of directed movement in bacteria
Bacteria possess specialised "receptors" or information-encoding devices, which are sensitive to light, chemicals, magnetic fields and so on. These receptors may or may not be activated, depending on the local environment. Bacterial receptors possess a short-term (three-second) "memory": they are activated by changes in their environment, rather than by the mere presence of their object. These receptors can alter the motion of bacteria.
A bacterium has two kinds of motion: directed movement (a "run", which occurs when a bacterium's rotary motors, or flagella, rotate in a counter-clockwise direction) and random tumbling (which occurs when a bacterium's flagella suddenly change direction and rotate clockwise). When the external section of a bacterial receptor recognizes and binds its target, a signal passes through the rest of the receptor and causes sequential changes in two proteins inside the bacterium. (This two-protein sensing system is found in all bacteria and in many other life-forms, but not in animals.) The first protein is called a kinase and sits next to the receptor. Normally, when there is no signal, this protein activates a second protein, the regulator, which interacts with the gear shift of a bacterium's flagella, causing them to turn clockwise and the bacterium to tumble randomly, about once every second.
However, when there is a signal from the receptor, the kinase cannot activate the regulator protein. Thus, the flagella continue to turn counterclockwise, and the bacterium, instead of tumbling, swims smoothly towards the target (Aegerter, 1997).
Case study 2: Directed movement in protoctista
Various kinds of directed movement, such as chemotaxis, thermotaxis (movement in response to heat), phototaxis and geotaxis (movement in response to gravity) are well-attested for protoctista (Martin and Gordon, 2001, p. 409). There does not seem to be any reason to treat these behaviours any differently from the directed movement of bacteria, as they do not appear to be flexible (see Conclusion F.3).
Case study 3: Directed movement in plants

The case of plants is philosophically interesting, because plants, unlike bacteria and protoctista, are incapable of locomotion: they stay where they are put. However, the possibility of cognition among plants should not be ruled out a priori. As Di Primio, Muller and Lengeler forcefully argue,
...[T]he fundamental thing [for an organism to behave] is not the ability to move to a new location, but the ability to modify itself (by developing effectors as needed), i.e. to respond appropriately to changing conditions (2000, p. 10).
Plants can move in a variety of ways. Whereas a taxis is defined as a movement of a cell in response to a stimulus, a tropism is the directional growth of a plant organ in response to a stimulus such as light (phototropism), water (hydrotropism), touch (thigmotropism) or gravity (geotropism), while a nastic movement (nasty) is a movement of a plant organ in response to stimuli, that is independent of the direction of the stimuli (e.g. the opening of flowers in response to changes in temperature or light, or the folding up of the leaves of the Mimosa plant when touched) (Isaacs, Daintith and Martin, 1999).
While conceding that the movement of plants is "either reduced to the cellular level or rather slow", Di Primio, Muller and Lengeler compare it favourably to that of animals:
Their abilities to perform undirected (so-called nasties) and directed movements (taxes, tropisms), however, is almost as complex and diverse, and certainly as purposeful, as those of animals. Plants have to solve the same problems as other organisms (2000, p. 10).
While Di Primio, Muller and Lengeler persuasively argue (2000, p. 10) that there appears to be no inherent reason why the absence of locomotion in plants, fungi and certain animals should preclude the possibility of cognition on their part, there is nothing in the literature, as far as I am aware, to suggest that plant movement is flexible, according to the definition used in this thesis, and as we have seen, research to date suggests that they are incapable of internally generated flexible behaviour (associative learning).
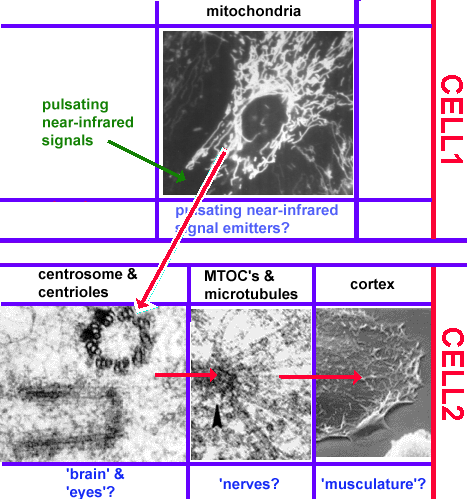
Albrecht-Buehler (2003a) believes that animal cells possess a kind of intelligence, and criticises the view that these cells are "rigidly operating chemical machines that derive their operating instructions internally from their genes and externally from chemicals and electrical signals emitted rigidly by other cells." He has made some intriguing claims regarding the centrosome, a spherical area near the nucleus of a cell, which (in animal cells but not in most plant cells) contains a pair of cylindrical structures called centrioles. Albrecht-Buehler claims that the centrosome is actually the control centre (or "brain") of an animal cell, while the centrioles function as the cell's "eyes". These "eyes" can detecting objects and other cells by pulsating near-infrared signals, and steer the cell towards their source. (The movement of animal cells differs from the phototactic behaviour of bacterial cells in several significant ways: bacteria, although light-sensitive, cannot see objects.) This means that cells can order and integrate a large amount of visual data.
Albrecht-Buehler believes that animal cells can navigate (see diagram), and that this ability is a manifestation of cell intelligence:
A moving cell has to operate its own body in sophisticated ways and, in addition, may have to navigate in space and time while dealing with numerous unforeseeable events, such as encounters with other cells and other objects that its genome could not possibly have anticipated. I think that cell motility, indeed, revealed cell intelligence.
Albrecht-Buehler's line of thinking here is that "the best place to start searching [for cell intelligence is] the field of cell movement."
If Albrecht-Buehler is correct, animal cells are capable of a special kind of navigation - visual navigation - which enables them to see objects from a long distance, whereas bacteria are only capable of tactile navigation.
Additionally, animal cells possess internal movement programs, and are programmed to turn at certain times in their lives at certain angles. In order to do this, they have to be able to measure times and angles. According to Albrecht-Buehler, cells' internal movement programs are not fixed: cells can over-ride them when circumstances warrant it. For instance, when travelling along a "road" made of tiny ridges, they sometimes leave the road, to investigate something "interesting". To do this, they use the distant "clue" to derive a new heading, and follow it. They are even programmed to seek information about their surroundings if they encounter more than one path they can follow. At intersections, the cells extend projections called pseudopodia in all directions, removing obstacles and often changing directions.
Individual cells can even co-ordinate their movements with one another:
PtK1 cells can migrate in groups that are much faster than the single cells... Some cells even appear to turn their bodies into a single leading edge of the group while the bodies of other cells turn themselves into a tail of the group... Therefore, the motility control systems appear to be able communicate with each other about about shape changes, direction and timing (Albrecht-Buehler, 2003b).
Case study: The nervous system and sensory capacities of cnidaria (jellyfish, sea anemones, corals and their relatives)
Relationship to other animals
The subkingdom Eumetazoa (so-called "true" animals, which excludes sponges) is made up of 33 phyla; most have tissues organized into organs and organ systems (World Biodiversity Database, 2000). The simplest of these phyla are the cnidaria (commonly known as coelenterates, including animals such as jellyfish, sea anemones, corals and freshwater hydra). Cnidaria have a body wall that is made up of only two layers of cells, like that of sponges; other "true" animals (eumetazoa) have three. Unlike sponges, cnidaria possess a basic kind of body symmetry: they are radially symmetrical.
Nervous system
Nervous systems and brains are unique to animals; they are not found in any other kingdom of organisms (World Biodiversity Database, 2000). Cnidaria have a rudimentary nervous system, with neurons positioned regularly over the surface of the animal. Each neuron is in contact with its neighbours. The propagation of a nerve impulse is not transmitted along a linear chain of neurons, but radiates from its point of origin (Abramson, 1994, p. 176).
Although cnidaria do not possess a central nervous system, let alone a brain, their nerve net permits rapid communication between cells (in some cases taking only milliseconds), over relatively long distances. In "simpler" animals, which lack neurons, communication can only occur between neighbouring cells. Prescott considers the nervous system they possess to be a major advance in the evolution of animals:
Whilst the most primitive metazoans [i.e. animals - V.T.], the sponges, lack neurons and respond only to direct stimulation (usually with a very slow, spreading contraction), cnidarians have quite complex nervous systems, composed, principally, of distributed nerve nets, and show both internally generated rhythmic behavior, and co-ordinated patterns of motor response to complex sensory stimuli.... Most of the neurophysiological features of more 'advanced' metazoan nervous systems are actually present at the cnidarian grade including multifunctional neurons, action potentials, synapses, and chemical neurotransmission... In ... the hydrozoan jellyfish, parts of the nerve net are fused to form longitudinal or circular tracts that allow very fast signal conduction and can support fast attack, escape, or defense reactions (2001, pp. 5 - 6).
According to Cotterill (2001, p. 5), most jellyfish exhibit a sluggish response to stimuli, but two types of jellyfish exhibit dual reponse patterns: slow feeding as well as the ability to rapidly escape from predators, which Cotterill considers to be a genuine autonomous reflex.
Some cnidaria - e.g. sea anemones and the jellyfish Aurelia aurita - possess a nerve net that is functionally divided into two relatively independent systems: one for feeding and the other for movement. In the jellyfish Aurelia aurita, the two systems make contact in neuron clusters called marginal ganglia. Each ganglion is a part of two networks: the network that controls feeding behaviour and a second network that regulates swimming. Because it is part of the swimming network, each ganglion has a regular beat, but it can also generate its own rhythm if isolated from the network. Other cnidaria, such as the jellyfish Aglantha digitale, have a different arrangement: "a single nerve net which can carry two different types of action potentials enabling either rapid escape swimming, or, slow rhythmic swimming for feeding" (Prescott, 2001, p. 6). Aglantha digitale has a giant axon with very fast conductance, so that a nervous impulse can traverse the circumference of the bell in a few milliseconds.
Senses in cnidaria
Cnidaria possess a variety of sensory capacities:
Many of the free-living cnidarians also possess light-sensitive and gravity-sensitive organs that allow behaviors such as orientation, sun compass navigation, and daily migration (Prescott, 2001, p. 6).
Cubozoans, or killer box jellyfish, are known to have complex eyes, similar in their basic design to those of vertebrates, despite the absence of a brain or central nervous system. The eyes connect into the neural network of the jellyfish, and there is evidence that they can see images. It has been suggested that for cubozoans, vision may play a role in feeding and reproductive behaviour:
Certain cubozoans are know to chase small fish and seize them with their tentacles. Further, many cubozoans exhibit complex sexual behaviors in which the males chase the females, grasp them with a tentacle, and subsequently inject packets of sperm into them. Vision may be important to the jellyfish for such complex behaviors (Martin, 2000).
Prescott considers the nervous system found in cnidaria to be a fundamental advance in the evolution of what he calls "action selection" or the problem of "resolving conflicts between competing behavioural alternatives" (2001, p. 1).
Prescott contends that the speed and co-ordination of the cnidarian response to stimuli represents a different kind of behaviour from that displayed by "simpler" animals such as sponges.
In some jellyfish, the nerve net is functionally divided into two relatively independent systems - one for feeding and the other for movement - which interact in neuron clusters. Others possess a single nerve net which can carry two different types of action potentials enabling either rapid escape swimming (to avoid predators), or, slow rhythmic swimming for feeding (Prescott, 2001, pp. 5-7).
Prescott likens this decentralised neural arrangement to the subsumption architecture described by Brooks (1986), which could be summed up in the adage, "You've got to crawl before you can walk". Rather than trying to build human intelligence into robots, Brooks designed a computer architecture that would display the range of functionality found in so-called "simple" life forms such as insects. Brooks argued that insects are "almost characterizable as deterministic machines". His architecture describes how an "agent" (be it a robot or a simple animal) can behave reliably in an unpredictable environment, despite having nothing more than cheap, small information processors, simple sensors and low memory. A Brooksian "agent" has no central control: it is hierarchically organised from the bottom up. Control is distributed between different components, making the "agent" better able to withstand damage (i.e. more robust). Behaviour patterns are hard-wired, and sensors and actuators (which produce movement) are closely coupled, to allow rapid response times. Co-ordination between the different components is ensured by built-in timers and by having behaviour modules that can inhibit one another. Simple behaviours combine to produce more complex patterns of behaviour (Laird, 1994). According to Prescott, the functional subdivision of the nerve net into two distinct circuits for feeding and movement, which is found in some jellyfish and sea anemones, resembles the Brooksian architecture proposed for some behaviour-based robots (2001, p. 6).
Can we regard an organism instantiating Brooks' architecture as having mental states? One could argue that some kind of primitive intelligence is required to co-ordinate the response of a motile, multicellular organism to a complex, unpredictable environment. This is the line of argument endorsed by Godfrey-Smith (2001), who has formulated his Environmental Complexity Thesis (ECT) to explain the evolution of cognition:
The function of cognition is to enable the agent to deal with environmental complexity.
The term "function" here is meant to be understood in a strong sense: the function of a trait is what it was selected for, in the course of its evolution. Godfrey-Smith defines "cognition" broadly, to include any kind of behaviour involving the coordination of actions with perceived environmental conditions. Naturally, he does not regard cognition as an on-off trait but as a trait that is found to some degree in all organisms - even those which are incapable of learning. However, we have argued that a mind-neutral intentional stance provides an adequate account of the adaptive behaviour found in bacteria, protoctista, plants and "simple" animals. The term "cognitive" is a superfluous here.
In any case, the specifications of Brooks' architecture make the ascription of mental states to a Brooksian "agent" redundant. A Brooksian "agent" has a very "low-tech" design. It has no internal model of the outside world - according to Brooks' design philosophy, such a system lacks the "computing" resources required to model a world which is dynamic and unpredictable. Additionally, a Brooksian "agent" does not engage in planning or learning of any kind. All of its behaviour is hard-wired and built-in, to ensure co-ordination and cope with unforeseen contingencies. In other words, its action patterns are fixed. If cnidaria do indeed behave like Brooksian "agents", then they cannot learn new ways of responding to unforeseen events. For reasons discussed above (Conclusion F.3), the ascription of cognitive mental states to cnidaria would then be redundant, as it would tell us nothing useful about their behaviour. A mind-neutral, goal-centred intentional stance would suffice.
Habituation has been documented in cnidaria (Encyclopedia Britannica, 1989). As we saw above, habituation does not qualify as "true" learning (Conclusion L.4).
I have not been able to locate any reports of experiments testing whether cnidaria are capable of associative learning. Clearly more work needs to be done in this area. For instance, one could repeatedly pair an unconditioned stimulus which causes a rapid escape response in hydrozoan jellyfish with the presence of light (a conditioned stimulus), and attempt to induce a conditioned rapid escape response to the presence of light alone.

The flatworm Pseudoceros diminuta. Courtesy of Bob Fenner, WetWebMedia.com.
Flatworms (platyhelminthes) are believed to be the most "primitive" phylum of worms, as they have the simplest central nervous systems. Flatworms also have an important evolutionary significance, as they are thought to resemble the common ancestor of all animals with bilateral symmetry (Prescott, 2001, p. 12).
Prescott (2001, p. 1) regards the appearance of the platyhelminthes in the fossil record (565 to 544 million years ago) as a breakthrough in the evolution of action selection, which he defines as the task of resolving conflicts between behavioural alternatives:
Animals of this sort are known to have been present in the [late] Precambrian [565 to 544 million years ago - V.T.], as demonstrated by the large number of trace fossils that have preserved the behaviour (e.g. foraging trails) though not the body forms of worm-like animals from that period. Simulation of these trace fossil patterns indicates a capacity for intelligent co-ordinated behaviors not unlike that demonstrated in some simple behavior-based robots (2001, p. 3).
While the first foraging trails to appear in the fossil record were rather inefficient and often criss-crossed each other (which is why they are often called "scribbles"), more regular, "meandering trails" had appeared by the end of the Precambrian, which looped back on themselves without crossing (Prescott, 2001, p. 12). For Prescott, these trails point to the emergence of the first creatures with brains.
Prescott invokes Occam's razor to argue that the fossil trails should be explained in the simplest possible way. He cites research by Raup and Seilacher (1969, cited in Prescott, 2001, pp. 12 - 13) that describes how computer simulations of the meandering trace fossil tracks can be generated by combining four simple behaviour mechanisms: stay close to previously formed tracks (thigmotaxis), avoid crossing existing tracks (phobotaxis), advance when the preceding conditions are not met; and make 180 degree U-turns at various intervals (strophotaxis), which is achieved by swapping control from one side of the body to the other at various intervals. While the first two behaviour patterns are reflexive mechanisms associated with an animal's peripheral sensors, and the third mechanism (advance) is merely a default, the behavioural component that suppresses signals from one side of the body while the other is active, functions as a centralised conflict-preventing mechanism, of vital importance to an organism with a brain and bilateral symmetry. Prescott considers this a breakthrough in the evolution of "action selection". Citing the work of researchers Koopowitz and Keenan (1982), he proposes that
the flatworm brain is primarily a mechanism that prevents the two sides of the body "from engaging in contradictory activities"... [T]he appearance of efficient foraging trails in the fossil records of the Precambrian may mark the point where centralized action selection mechanisms [i.e. brains - V.T.] had evolved to take control over peripheral reflex systems (2001, p. 15).
(This section has no Appendix material.)
Beisecker's interpretation of blocking

For David Beisecker the defining quality of beliefs is that they can be correct or mistaken. According to Beisecker, animals capable of operant conditioning should be regarded as having beliefs because not only are they educable, but they can make errors which they subsequently try to rectify when they realise that their expectations were mistaken.
Beisecker has proposed blocking as an example of behaviour that expectation-generating animals would engage in. I shall discuss this particular claim in detail, as it is a highly unusual prediction of one model of associative learning - the Rescorla-Wagner model - whose central ideas are usually expounded using mentalistic terminology.
While the Rescorla-Wagner model is not able to explain all phenomena connected with classical conditioning, it is still regarded as "the 'best' theory of classical conditioning" (Jackson, 2002). Its basic principle is that "the amount of conditioning depends on how surprising the association between the CS and US is. Surprise determines not only if conditioning occurs but how much conditioning occurs. The more unexpected or surprising the US, the more conditioning will occur" (Lipp, 1998, italics mine). Surprise surely qualifies as a mental state.
Blocking, which is readily explained by the model, has been interpreted by some learning theorists as evidence that animals form expectations and hence have mental states. Beisecker describes how it works:
Several learning theorists have argued that the apparent educability of some creatures is best explained in terms of the adjustment of "expectation-like" structures mediating between sensory input and behavioral output. For example, expectations are a reasonable explanation for the blocking phenomena often observed in actual creatures. Animals that have been trained to associate a conditioned stimulus with an unconditioned stimulus will subsequently fail to associate other stimuli with the unconditioned stimulus, when the latter are presented along with the original conditioned stimulus. For example, rats that have been trained to associate a bell tone with an electric shock will not come to associate a red light with a shock, as long as the red light is consistently paired with the bell tone. The prior conditioning prevents (or "blocks") subsequent conditioning to other, co-varying stimuli. If learning were merely a function of the frequency of stimulus-pairing, then one would expect the animal to become conditioned to the new stimulus as well. One would expect the rats eventually to associate the red light with a shock, as indeed they do when they aren't subjected to the earlier training. Many learning theorists have argued that the failure of previously conditioned animals to become conditioned to the new stimulus arises because the animal already uses the original conditioned stimulus to successfully predict the occurrence of the unconditioned stimulus. When a previously conditioned rat encounters the compound tone and light stimulus, it expects that the shock will occur (because it heard the bell tone), and so the subsequent shock isn't a surprise. Since events are as they were expected to be (they were not novel), there is no pressure to develop new associations, hence no subsequent conditioning to the light. Thus these theorists conclude that the rats are responding to surprise, to things not being as they expected them to be (Beisecker, 1999, pp. 298-299, italics mine).
The ability to form expectations is certainly a sufficient condition for having mental states, and if Beisecker's account of belief is correct, it is a necessary condition as well. If blocking indicates the presence of expectations, then can we use the phenomenon of blocking to distinguish those animals with minds from those without?
Do all educable animals display blocking?
There are two good reasons for caution here. First, associative learning is widespread in the animal kingdom: even simple worms have been shown to be capable of it. The evidence for operant conditioning is less clear, but it is well-documented for many kinds of insects, and we have already discussed tentative evidence that worms such as C. elegans may be capable of it. However, most kinds of animals that are capable of operant conditioning (especially insects) have not yet been shown to exhibit blocking.
I emailed Bill Nuttley, a neurobiologist whose specialty is the nematode worm C. elegans, and asked him whether blocking had been confirmed or disconfirmed in this worm. I received the following reply (Nuttley, personal email, 18 July 2003):
As for blocking, a student has been looking at that and although the trends are all encouraging and the data looks pretty good, we fall just short of making stats. A few changes to the protocol and I think we will be able to demonstrate blocking, but so far no.
In response to a query as to whether blocking has been confirmed yet in any invertebrates, Dr Bjorn Brembs (personal email, 22 December 2002) wrote:
As far as I have heard, the jury is still out, whether there is blocking, although those that have found it still claim that there is no dispute about their data. There are a few finds, but alternative explanations have not been ruled out, yet. So far, blocking, if it is there, is definitely not as universal and general as in vertebrates, at the least.
The occurrence of blocking even in so-called "higher" invertebrates (honeybees) remains very controversial.
If Beisecker wishes to propose blocking as a litmus test of cognitive mental states, then he will have to limit these states to invertebrates.
Are there any non-cognitive interpretations of blocking?
A second reason for caution is that blocking may turn out to be explicable in non-mentalistic terms, in any case. As one neurobiologist who does research in this field commented (Menzel, personal email communication, 21 July 2003):
Expectation is a term that I am also using in these papers but not with a high order cognitive meaning. It is my impression that scientists in my field are considering expectation as a function that results from former learning and creates a status of retrieved memory without implications about any separate cognitive state other than memory retrieval. There are even concepts that try to explain blocking as a function of peripheral sensory integration.
We have already seen that sensory capacities and memory can be explained by adopting a goal-centred intentional stance, without the need for a richer, mentalistic account. If many scientists believe that these capacities are sufficient to explain blocking, then (pace Beisecker) it would be unwise to invoke blocking as evidence of mental states.