     |
http://seit.humber.ac.uk/~tharris/EGF539/Lectures/silicon_nitride.htm
Silicon Nitride (Si3N4)
Silicon nitride is an industrial ceramic that has high resistance to thermal shock and an ability to maintain a high level of hardness
and fracture toughness at elevated temperatures.
These properties make silicon nitride ideal for applications such as all ceramic gas turbine blades, replacements for metallic parts
in internal combustion engines and for cutting tools.
Silicon nitride is a covalently bonded ceramic that exists in two forms – a and b. Both structures have hexagonal unit cells.
The unit cell of the a structure has a ratio c/a =0.7 whilst the b form has c/a = 0.37.
The larger a unit cell produces a distorted pyramidal structure with large internal strains.
Compared with the b form, which is more symmetrical, the a form is unstable e.g.
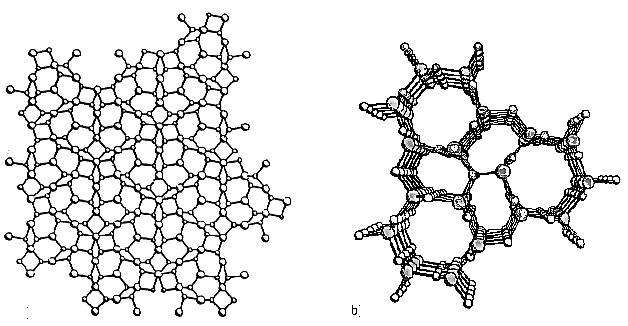
Figure 1. Molecular structure of Silicon Nitride a (left) and b (right).
It is therefore desirable to have a b – silicon nitride microstructure.
Mechanical Properties.
The formation of dense silicon nitride using metal oxides means that it is rare
to find a single phase b-silicon nitride ceramic.
The oxynitride liquid forms an intergranular glassy or amorphous phase. This
glassy phase can lead to a reduction in mechanical
properties at high temperatures, with the glassy phases formed using different
metal oxides having different melting temperatures.
Therefore it is important to take processing routes and additives into account
when considering silicon nitride properties.
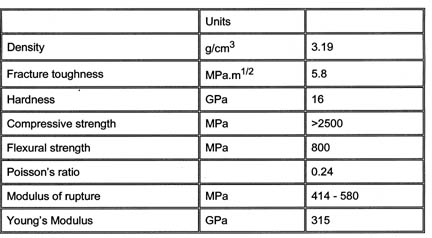
Figure 2. Some of the mechanical properties of silicon nitride.
There are two main features that bear heavily on the mechanical properties of
a silicon nitride:
1)grain size
2)aspect ratio of the grains.
Where the aspect ratio is the ratio of grain length to grain diameter.
Fracture toughness
Fracture in silicon nitride is dominated by the glassy phase with most cracks
travelling through this weaker material.
The long b-silicon nitride grains provide a high resistance to crack growth,
either by making the cracks travel by longer paths or by
deflecting the crack.
The grains can be encouraged to grow by increasing the hot pressing time. Figure
6 shows the effect of processing route on
fracture toughness values.
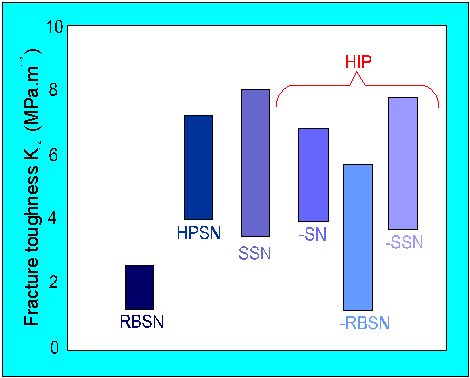
Figure 3. K1C values for various types of silicon nitride.
Increased grain growth causes the glassy phase to be squeezed out from beneath
the grains producing greater interfacial bonding.
Thus the fracture mechanism changes to transgranular and needs greater amounts
of energy for cracks to propagate.
It has also been shown that adding large amounts of certain metal oxides can
also increase fracture toughness values e.g.
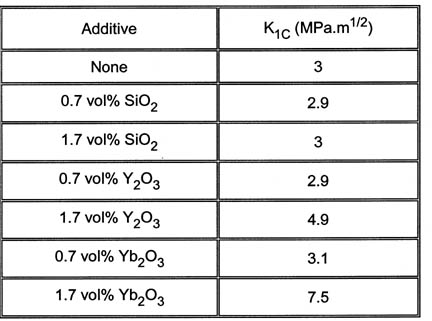
Figure 4. Variation in K1C with addition of sintering aids.
High temperature fracture toughness
At high temperatures fracture toughness is dominated by
There is evidence to suggest that there is a mechanism change in fracture at
high temperatures e.g.
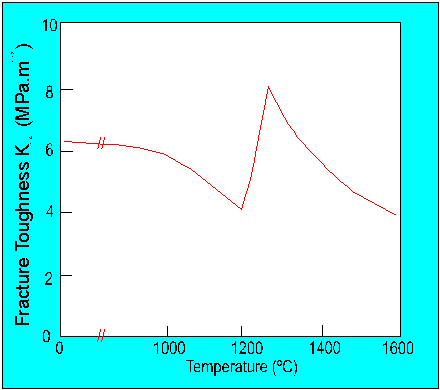
Figure 5. The relationship between K1C and temperature for silicon nitride.
Hardness
The hardness of silicon nitride is also dependent on the processing route and
the addition of metal oxides.
Hardness values for silicon nitride will vary with direction of pressing producing
quite drastic anisotropic variations in hardness.
The range of values obtained depend on the processing route.
High hardness and low anisotropy in hardness can be obtained by HIPing because
of the density obtained and small equiaxed
grains with strong interfacial bonding.
The amount of glassy phase present also will affect the values of hardness obtained
with larger amounts of this residual phase
causing a reduction in hardness.
Sialons
Sialons are silicon nitride ceramics alloyed with metal oxides.
For b-silicon nitride ceramics alloyed with alumina (Al2O3) the alloy is designated as b’-sialon (silicon, aluminium, oxygen, nitrogen).
In these materials 2/3rds of the silicon in b-silicon nitride can easily be replaced by aluminium without changing the structure whilst the equivalent amount of nitrogen is replaced by oxygen.
Because b-sialons have the same atomic arrangement as b-silicon nitride its mechanical properties are also similar.
Artefacts based on these materials are sintered using additives which produce microstructures containing grain boundary phases of either nitrogen-oxygen glass or yttrium-aluminium garnet (YAG).
Alloys of a-silicon nitride and oxides of lithium (Li), Calcium (Ca) and Yttrium (Y) produce a-sialons.
As with preparations of silicon nitride it is quite common to find microstructures with mixtures of a and b sialons.
To determine the amount of a and b present the Vickers hardness technique can be used, as there is a linear correlation between b and hardness e.g.
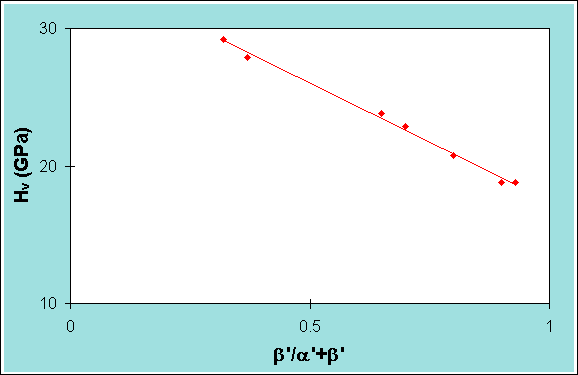
Figure 6. The linear dependence of hardness of the b’-sialon content of a sialon artifact.
A third family of sialons exist based on the family Si2N2O structure. These are known as the o’ sialons.
These materials are generally used high wear and high temperature applications.