The wave function of the hydrogen atom in the (n, l, ml) = (2,1,0) state, y(r, q, f), is
 .
.Note that the wave function has no f dependence.
The figure below is a grayscale contour plot of this function. Dark means high density.
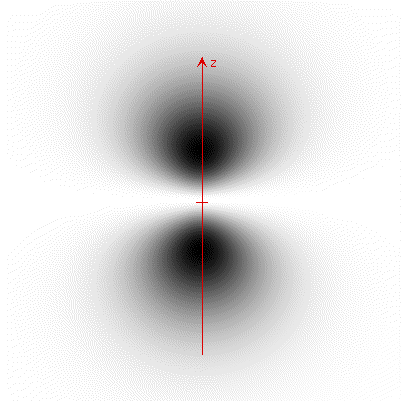
a) Along which axis is the probability density greatest? Answer x = 1, y = 2, or z = 3.
The probability density is greatest along the axis.
b) On the z axis, at z = ao, what is the probability density, |y|2? You must give a symbolic answer, expressed in terms of the appropriate combination of h, c, ao, and numbers (pi, etc). Note that we have chosen convenient units: per ao3.
Probability density = per ao3.
c) Consider all points a distance r = ao from the origin as (the point in part b is one such point). At what polar angle, q, between 0 and 90 degrees is the probability density one-half of the value in part b?
q = degrees.