The ground state wave function for the hydrogen atom is y(r, q, f) = (pao3)-1/2 exp(-r/ao).
a) Find the probability that the electron occupies a small volume
DV = (ao)3 at three radii:
r = 0, ao, and 2ao.
A plot of the probablity density (in units of probability per ao3) is
shown below.
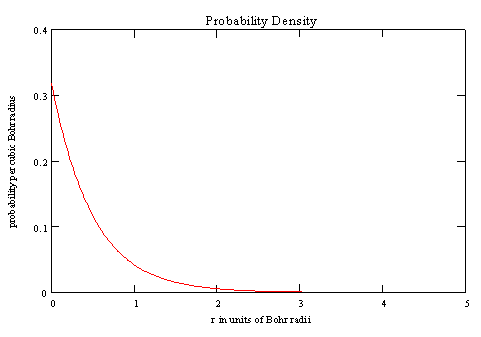
The probability of occupying DV near r = 0 is
The probability of occupying DV near r = ao is
The probability of occupying DV near r = 2ao
is
b) Find the probability that the electron occupies a thin
spherical shell of thickness Dr = ao,
at three radii: r = 0, ao, and 2ao.
A plot of the radial probablity density (in units of probablity per ao) is
shown below.
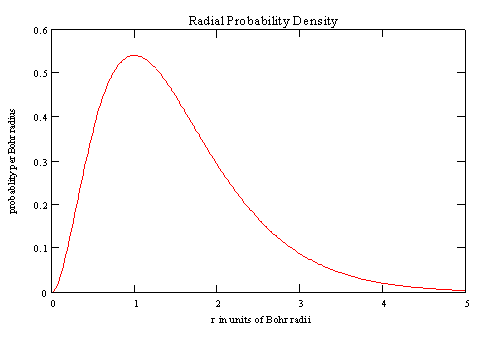
The probability of occupying a Dr-thick shell
at r = 0 is
The probability of occupying a Dr-thick shell
at r = ao is
The probability of occupying a Dr-thick shell
at r = 2ao is
NOTE: This page is a kind contribution of "Snot Noodle"