Now we get a bit messy with the experimenting. Aside from a few spills, this one proved a point, which explains WHY today's IC engines are so inefficient.
A test tube was filled 1/4 way up with pump gasoline, and a PVC hose was inserted into the end. The other end of the hose was placed inside an aluminum tray with an aluminum block covering over it. The test tube was heated with a small propane flame, as seen below. The flame had to be kept very small at first, because if the foam rose too far, it "leaked" down the hose into the tray. But when the foam was not leaking out, the vapours that rose through the hose were rapidly condensing just inches from the mouth of the test tube. No vapours remained at the end of the hose.
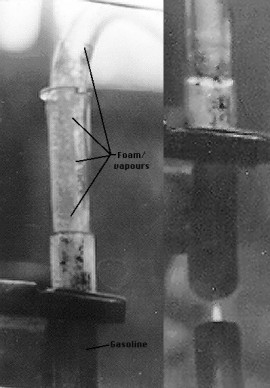
Second "stage" of the fuel boiled off as the light stuff disappeared from the sample. This "stage" of the fuel did not create quite as much foam, but it took more heat to drive it off. It too condensed back, but some of the vapours were seen turning to aerosol inside the hose, something it didn't do before
Finally, the heavier parts created a lot of aerosol "milk" inside the hose and were harder to recondense. But it didn't create foam. Before too much of this heavy junk could go off into the distilled stuff, the heat was removed. The test tube was drained of the heavy stuff, refilled 1/4 way with another sample, and re-distilled. The process was repeated until about a pint of distilled fuel was recovered.
The distilled fuel was put through yet another distillation process, this time to separate the lightest of the gas from the mid-range portions. Only a small amount of heat was used to drive the light stuff off, and it was clearly visible when it was all gone, as the foam descended and aerosol began to form inside the hose.
In the end, three fractions of the gasoline resulted. The heaviest portion was oily, dark, and had a strong odour. The middle "class" was lighter, had less odour, and was more volatile. But the light stuff was amazing...it was almost white with barely any odour, and seemed to be more combustible than regular gasoline. However, it burned pretty much just as cruddy as the original sample before distillation.
Pictures of the samples will be up soon, but I wanted them in colour with maximum quality, so a better camera is required
Another experiment proved just deadly-sweet. Motor oil (10 W 30) in a jar with a spark plug in it. The plug had a gap of less than 1/16", and was operating off the 555 timer array. When it was turned on, sparks were generated under the liquid oil. But the most amazing part is, there were gases rising through the oil, and the gases were not oil vapours (at least they didn't smell like it). So what was it? It certainly didn't behave like oil in the presence of a flame...it popped. Oil barely burns at room temp, but this stuff was surprising.
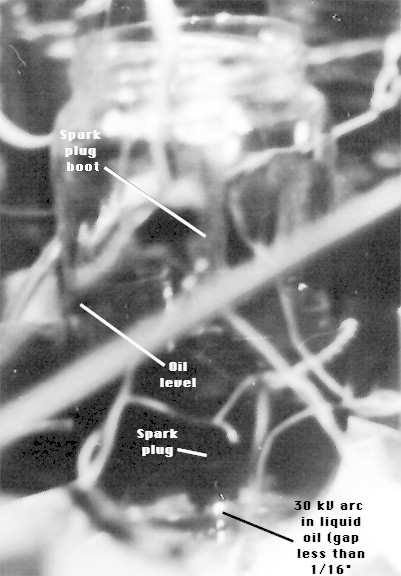
More tests will be conducted to determine the practicality of this fuel. The test will also be done with gasoline. Stay tuned...
This page still under construction...