|
Electromagnetic Radiation |
Forms
of electromagnetic radiation
Gamma Rays
Six years after the discovery of
radioactivity (1896) by Henri Becquerel of France, the New Zealand-born
British physicist Ernest Rutherford found that three different kinds of
radiation are emitted in the decay of radioactive substances; these he
called alpha, beta, and gamma rays in sequence of their ability to penetrate
matter. The alpha particles were found to be identical with the nuclei of
helium atoms and the beta rays were identified as electrons. In 1912 it was
shown that the much more penetrating gamma rays have all the properties of
very energetic electromagnetic radiation, or photons. Gamma-ray photons are
between 10,000 and 10,000,000 times more energetic than the photons of
visible light when they originate from radioactive atomic nuclei. Gamma rays
with a million million times higher energy make up a very small part of the
cosmic rays that reach the Earth from supernovae or from other galaxies. The
origin of the most energetic gamma rays is not yet known.
During radioactive decay, an unstable
nucleus usually emits alpha particles, electrons, gamma rays, and neutrinos
spontaneously. In nuclear fission, the unstable nucleus breaks into
fragments, which are themselves complex nuclei, along with such particles as
neutrons and protons. The resultant stable nuclei or nuclear fragments are
usually in a highly excited state and then reach their low-energy ground
state by emitting one or more gamma rays. Such a decay scheme is shown
schematically in Figure 7.
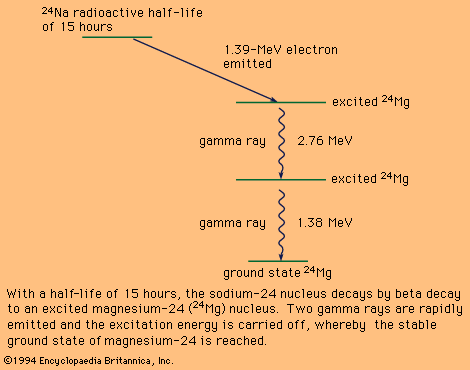
Decay scheme of a radioactive sodium-24 (24Na) nucleus.
With a half-life of 15 for the unstable nucleus sodium-24
(24Na). Much of what is known about the internal structure and energies of
nuclei has been obtained from the emission or resonant absorption of gamma
rays by nuclei. Absorption of gamma rays by nuclei can cause them to eject
neutrons or alpha particles or it can even split a nucleus like a bursting
bubble in what is called photodisintegration. A gamma particle hitting a
hydrogen nucleus (that is, a proton), for example, produces a positive
pi-meson and a neutron or a neutral pi-meson and a proton. Neutral
pi-mesons, in turn, have a very brief mean life of 1.8 10-16 second and
decay into two gamma rays of energy h 70 MeV. When an energetic gamma ray h
> 1.02 MeV passes a nucleus, it may disappear while creating an
electron-positron pair. Gamma photons interact with matter by discrete
elementary processes that include resonant absorption, photodisintegration,
ionization, scattering (Compton scattering), or pair production.
Gamma rays are detected by their ability to ionize gas atoms or to create
electron-hole pairs in semiconductors or insulators. By counting the rate of
charge pulses or voltage pulses or by measuring the scintillation of the
light emitted by the subsequently recombining electron-hole pairs, one can
determine the number and energy of gamma rays striking an ionization
detector or scintillation counter.
Both the specific energy of the gamma-ray photon emitted as well as the
half-life of the specific radioactive decay process that yields the photon
identify the type of nuclei at hand and their concentrations. By bombarding
stable nuclei with neutrons, one can artificially convert more than 70
different stable nuclei into radioactive nuclei and use their characteristic
gamma emission for purposes of identification, for impurity analysis of
metallurgical specimens (neutron-activation analysis), or as radioactive
tracers with which to determine the functions or malfunctions of human
organs, to follow the life cycles of organisms, or to determine the effects
of chemicals on biological systems and plants.
The great penetrating power of gamma rays stems from the fact that they have
no electric charge and thus do not interact with matter as strongly as do
charged particles. Because of their penetrating power gamma rays can be used
for radiographing holes and defects in metal castings and other structural
parts. At the same time, this property makes gamma rays extremely hazardous.
The lethal effect of this form of ionizing radiation makes it useful for
sterilizing medical supplies that cannot be sanitized by boiling or for
killing organisms that cause food spoilage. More than 50 percent of the
ionizing radiation to which humans are exposed comes from natural radon gas,
which is an end product of the radioactive decay chain of natural
radioactive substances in minerals. Radon escapes from the ground and enters
the environment in varying amounts.
|