|
Electromagnetic Radiation |
The electromagnetic spectrum
The
brief account of familiar phenomena given above surveyed electromagnetic
radiation from small frequencies (long wave radios) to exceedingly high
values of (gamma rays). Going from the values of radio waves to those of
visible light is like comparing the thickness of this page with the distance
of the Earth from the Sun, which represents an increase by a factor of a
million billion. Similarly, going from the values of visible light to the
very much larger ones of gamma rays represents another increase in frequency
by a factor of a million billion. This extremely large range of values,
called the electromagnetic spectrum, is shown in Figure 1
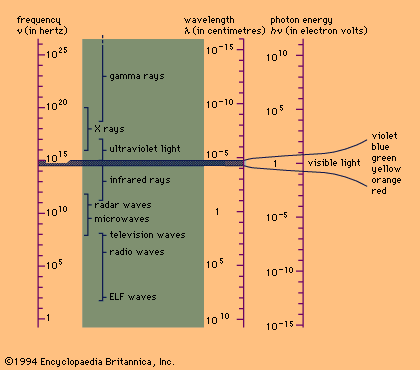
Electromagnetic spectrum. The small visible range (shaded) is shown enlarged
at the..., together with the common names used for its various parts, or
regions.
The
number is shared by both the classical and the modern interpretation of
electromagnetic radiation. In classical language, is the frequency of the
temporal changes in an electromagnetic wave. The frequency of a wave is
related to its speed c and wavelength in the following way. If 10 complete
waves pass by in one second, one observes 10 wriggles, and one says that the
frequency of such a wave is = 10 cycles per second (10 hertz [Hz]). If the
wavelength of the wave is, say, = 3 centimetres, then it is clear that a
wave train 30 centimetres long has passed in that one second to produce the
10 wriggles that were observed. Thus, the speed of the wave is 30
centimetres per second, and one notes that in general the speed is c = . The
speed of electromagnetic radiation of all kinds is the same universal
constant that is defined to be exactly c = 299,792,458 metres per second
(186,282 miles per second). The wavelengths of the classical electromagnetic
waves in free space calculated from c = are also shown on the spectrum in
Figure 1 , as is the energy h of modern-day photons. One commonly uses as
the unit of energy electron volt (eV), which is the energy that can be given
to an electron by a one-volt battery. It is clear that the range of
wavelengths and of photon energies h are equally as large as the spectrum of
values.
Because the wavelengths and energy quanta h of electromagnetic radiation of
the various parts of the spectrum are so different in magnitude, the sources
of the radiations, the interactions with matter, and the detectors employed
are correspondingly different. This is why the same electromagnetic
radiation is called by different names in various regions of the spectrum.
In
spite of these obvious differences of scale, all forms of electromagnetic
radiation obey certain general rules that are well understood and that allow
one to calculate with very high precision their properties and interactions
with charged particles in atoms, molecules, and large objects.
Electromagnetic radiation is, classically speaking, a wave of electric and
magnetic fields propagating at the speed of light c through empty space. In
this wave the electric and magnetic fields change their magnitude and
direction each second. This rate of change is the frequency measured in
cycles per second--namely, in hertz. The electric and magnetic fields are
always perpendicular to one another and at right angles to the direction of
propagation, as shown in Figure 2

Radiation fields in which vectors and are perpendicular to each other and to
the....
There is as much energy carried by the electric component of the wave as by
the magnetic component, and the energy is proportional to the square of the
field strength.
|