We all use water everyday in many different ways, but we almost never think of it as individual atoms bonded together to make molecules. These molecules then bond together to get different volumes of water like gallons, liters, milliliters, etc. To really understand water, you have to go much smaller than these measurments, you have to go to atoms and molecules.
Each molecule of water is made up of one oxygen atom and two hydrogen atoms. These bond together with a covalent bond. A covalent bond is made when two atoms share their electrons with each other. In this case, the oxygen shares two electrons with one hydrogen atom and two with the other hydrogen atom. The electrons that are shared are in the atomís valence (or outer) shell. Hydrogen atoms only have one electron, so that is one of the electrons shared with the oxygen atom. On the other hand, oxygen atoms have six electrons in their valence shell and they need two more to make a full shell. To get these two atoms, it shares one with one hydrogen atom and another with another hydrogen atom. This also gives the hydrogen atoms full valence shells so they become less reactive. This only makes one molecule of water. To have volumes of water the molecules need to bond together.
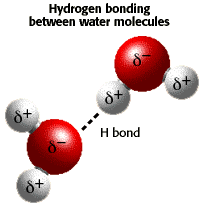
The water molecules create a hydrogen bond between each other so that it creates an amount of water that we can actually see. A hydrogen bond is formed between two polar molecules, which are molecules that have a side that has a positive charge and a side with a negative charge. The charges are different because of the difference in the number of electrons on that side. On a water molecule, the side where the hydrogen atoms has a positive charge because all of the electrons are around the oxygen atom. The oxygen atom has a negitive charge becasue most of its molecules are gathered on that side because they were repelled by the hydrogen atoms. Different charges attract, so the oxygen atom of one molecules attracts to a hydrogen atom of another molecules, forming a hydrogen bond, which is more loosly bound than a covalent bond.
This is a picture of two water molecules connected by a hydrogen bond.
http://www.biology.arizona.edu/biochemistry/tutorials/chemistry/page3.html