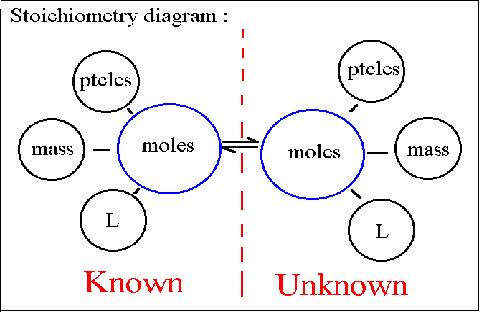
Stoichiometry is mass and quantity relationships among reactants and products in a chemical reaction. You can use stoichiometry to find how much a reaction can produce. You can use stoichiometry to answer these types of questions:
What is the mass in grams of N mols of the element X?
N mols. x atomic mass in grams of element X = _____ grams X 1 mol. of element X
How many moles of X are in N grams of element X?
N grams x 1 mol of element X = _____ mols. X Atomic mass in grams of X
How many moles of element X are in N atoms of element X?
N atoms x 1 mol X = _____ atoms X 6.022 x 1023 atoms
How many atoms of element X are in N mols. of element X?
N mols. X x 6.022 x 1023 atoms = _____ atoms X 1 mol. X
What is the mass in grams of N atoms of element X?
N atoms x atomic mass in grams of element X = _____ grams X 6.022 x 1023 atoms
How many atoms of element X are in N grams of element X?
N grams X x 6.022 x 1023 = _____ atoms X Atomic mass in grams of X
What mass of X2 contains the same number of atoms as N grams of element X1?
N grams X1 x atomic mass in grams of element X2 = _____ grams X2 atomic mass in grams of element X1

Main menu
Topic 5: Chemical Equations
Topic 7: Causes of Change
Credits and Links