Atrial
Infarction
A number of ECG clues to the diagnosis of atrial infarction have been suggested, including localized deviations of the PR segment (e.g., PR elevation in V5 or V6), changes in P wave morphology, and atrial arrhythmias. However, the sensitivity and specificity of these signs are limited. Diffuse PR segment changes (PR elevation in aVr with depression in the inferolateral leads) with acute infarction usually indicate concomitant pericarditis (see below).
ECG Differential Diagnosis of Ischemia
and Infarction
The ECG has important limitations in both sensitivity and specificity in the diagnosis of coronary syndromes. An initially normal ECG does not exclude ischemia or even acute infarction. However, a normal ECG throughout the course of an alleged acute infarct is distinctly uncommon. As a result, prolonged chest pain without diagnostic ECG changes should always prompt a careful search for noncoronary causes of chest pain. Pathological Q waves may be absent even in patients with depressed left ventricular function caused by severe coronary disease and a previous infarct. As noted, the diagnosis of acute or chronic infarction can be completely masked by ventricular conduction disturbances, especially those resulting from LBBB, as well as ventricular pacing and WPW preexcitation. On the other hand, diagnostic confusion can arise because Q waves, ST elevation, ST depression, tall positive T waves, and deep T wave inversion can be seen in a wide variety of noncoronary settings.
Q waves simulating coronary artery disease can be related to one (or a combination) of the following four factors : (1) physiological or positional variants, (2) altered ventricular conduction, (3) ventricular enlargement, and (4) myocardial damage or replacement. Depending on the electrical axis, prominent Q waves (as part of QS- or QR-type complexes) can also appear in the limb leads (aVl with a vertical axis and III and aVf with a horizontal axis). A QS complex can appear in lead V1 as a normal variant and rarely in leads V1 and V2. Prominent Q waves can be associated with a variety of other positional factors that alter the orientation of the heart vis-ŕ-vis a given lead axis. Poor R wave progression, sometimes with actual QS waves, can be due solely to improper placement of chest electrodes above their usual position. In dextrocardia, provided that no underlying structural abnormalities are present, normal R wave progression can be restored by recording leads V2 to V6 on the right side of the chest. A rightward mediastinal shift in left pneumothorax can contribute to the apparent loss of left precordial R waves. Other positional factors associated with poor R wave progression include pectus excavatum, congenitally corrected transposition of the great vessels, and congenital absence of the left pericardium.
An intrinsic change in the sequence of ventricular depolarization can lead to pathological, noninfarct Q waves. The two most important conduction disturbances associated with pseudoinfarct Q waves are LBBB and the WPW preexcitation patterns. With LBBB, QS complexes can appear in the right precordial to midprecordial leads and occasionally in one or more of leads II, III, and aVf.. Depending on the location of the bypass tract, WPW preexcitation can mimic anteroseptal, lateral, or inferior-posterior infarction. LAFB is often cited as a cause of anteroseptal infarct patterns. However, LAFB has only minor effects on the QRS complex in horizontal plane leads. Probably the most common findings are relatively prominent S waves in leads V5 and V6. Poor R wave progression is not a routine feature of LAFB, although some authors have reported minuscule q waves in leads V1 to V3 in this setting. These small q waves can become more apparent if the leads are recorded one interspace above their usual position and disappear in leads one interspace below their usual position. However, as a general clinical rule, prominent Q waves (as part of QS or QR complexes) in the right precordial to midprecordial leads should not be attributed to LAFB alone.
In contrast, poor R wave progression is commonly observed with LVH and with acute or chronic right ventricular overload. Q waves in such settings can reflect a variety of mechanisms, including a change in the balance of early ventricular depolarization forces and altered cardiac geometry and position. A marked loss of R wave voltage, sometimes with frank Q waves from V1 to the lateral chest leads, may be seen with chronic obstructive pulmonary disease. The presence of low limb voltage and P pulmonale can serve as additional diagnostic clues. This loss of R wave progression may, in part, reflect right ventricular dilation. Furthermore, downward displacement of the heart in an emphysematous chest may play a major role in the genesis of poor R wave progression in this syndrome. Partial or complete normalization of R wave progression may be achieved in such cases simply by recording the chest leads an interspace lower than usual .
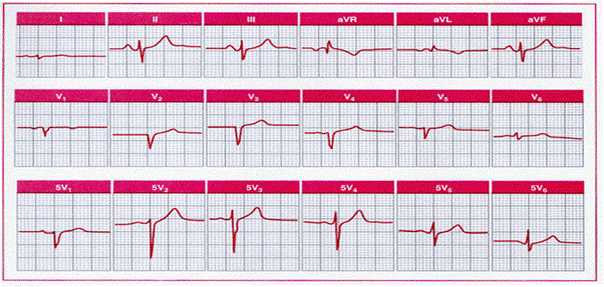
Pulmonary emphysema
simulating anterior infarction in a 58-year-old man with no clinical evidence of
coronary disease. Note the relative normalization of R wave progression with
placement of the chest leads an interspace below their usual position (5V1,
5V2,
and so forth).
A variety of pseudoinfarct patterns can occur with acute cor pulmonale caused by pulmonary embolism. Acute right ventricular overload in this setting can cause poor R wave progression and sometimes right precordial to midprecordial T wave inversion (right ventricular “strain”) mimicking anterior infarction. The classic S1Q3T3 pattern can occur but is neither sensitive nor specific. A prominent Q wave (usually as part of a QR complex) can also occur in lead aVf along with this pattern. However, acute right overload by itself does not cause a pathological Q wave in lead II. Right heart overload, acute or chronic, may also be associated with a QR complex in lead V1 and simulate anteroseptal infarction.

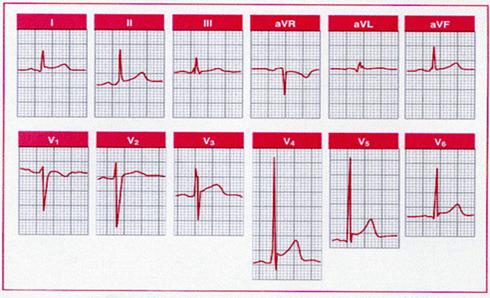
Acute cor pulmonale
secondary to pulmonary embolism simulating inferior and anterior infarction.
This tracing exemplifies the classic pseudoinfarct patterns sometimes seen: an S1
Q3
T3,
a QR in V1 with poor R wave
progression in the right precordial leads (“clockwise rotation”), and right
precordial to midprecordial T wave inversion (V1
to V4
). Sinus tachycardia is also
present. The S1 Q3
pattern is usually associated with a
QR or QS complex, but not an rS, in aVr.
Furthermore, acute cor pulmonale per se does not cause prominent Q waves in II
(only in III and aVf ).
Pseudoinfarct patterns are an important finding in patients with hypertrophic cardiomyopathy, and the ECG can simulate anterior, inferior, posterior, or lateral infarction. The pathogenesis of depolarization abnormalities in this cardiomyopathy is not certain. Prominent inferolateral Q waves (II, III, aVf, and V4 to V6) and tall right precordial R waves are probably related to increased depolarization forces generated by the markedly hypertrophied septum . Abnormal septal depolarization may also contribute to the bizarre QRS complexes.
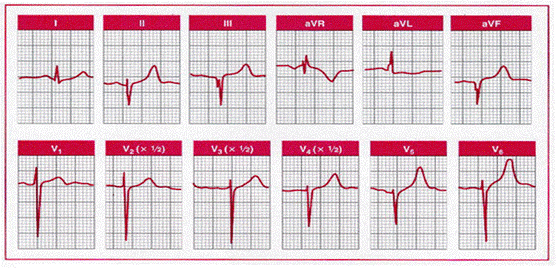
Hypertrophic
cardiomyopathy simulating inferolateral infarction. This 11-year-old girl had a
family history of hypertrophic cardiomyopathy. Note the W-shaped QS waves and
the QRS complexes in the inferior and lateral precordial leads.
Loss of electromotive force associated with myocardial
necrosis contributes to R wave loss and Q wave formation in myocardial
infarction. This mechanism of Q wave pathogenesis, however, is not specific for
coronary artery disease with infarction. Any process, acute or chronic, that
causes sufficient loss of regional electromotive potential can result in Q
waves. For example, replacement of myocardial tissue by electrically inert
material such as amyloid or tumor may cause noninfarction Q waves. A variety of
dilated cardiomyopathies associated with extensive myocardial fibrosis may be
characterized by pseudoinfarct patterns. Ventricular hypertrophy can also
contribute to Q wave pathogenesis in this setting. Finally, Q waves caused by
myocardial injury, whether ischemic or nonischemic in origin, can appear
transiently and do not necessarily signify irreversible heart muscle damage.
Severe ischemia can cause regional loss of electromotive potential without
actual cell death (“electrical stunning” phe nomenon).
Transient conduction disturbances can also cause alterations in ventricular
activation and result in noninfarctional Q waves. In some cases, transient Q
waves may represent unmasking of a prior Q wave infarct. New, but transient Q
waves have been described with severe hypotension from a variety of causes, as
well as with tachyarrhythmias, myocarditis, Prinzmetal's angina, protracted
hypoglycemia, phosphorus poisoning, and hyperkalemia.
ST-T Changes Simulating Ischemia
Top, Acute pericarditis is often characterized by two
apparent injury currents: one atrial, the other ventricular. The atrial injury
current vector (ST a ) is
usually directed upward and to the right and produces PR segment elevation in aVr
with reciprocal PR depression in II, V5,
and V6. The ventricular injury
current (ST V ) is directed
downward and to the left, associated with ST elevation in II, V5,
and V6 with reciprocal ST
depression in aVr. This
characteristic PR-ST segment discordance is illustrated in the Bottom, Note the
diffuse distribution of ST segment elevation in acute pericarditis (e.g., I, II,
and V2 through V6,
with reciprocal changes in aVr and
perhaps minimally in V1 ). Note
the PR segment elevation in aVr

|
A variety of factors such as digitalis, ventricular
hypertrophy, hypokalemia, and hyperventilation can cause ST segment depression
mimicking subendocardial ischemia. Similarly, tall positive T waves do not
invariably represent hyperacute ischemic changes but can reflect normal
variants, hyperkalemia, cerebrovascular injury, and left ventricular volume
loads resulting from mitral or aortic regurgitation, among other causes. ST
elevation and tall positive T waves are also common findings in leads V1
and V2 with LBBB or LVH patterns.
 In
addition, tall T waves may be seen occasionally in the left chest leads with LVH,
especially with volume (diastolic) overload syndrome .
In
addition, tall T waves may be seen occasionally in the left chest leads with LVH,
especially with volume (diastolic) overload syndrome .
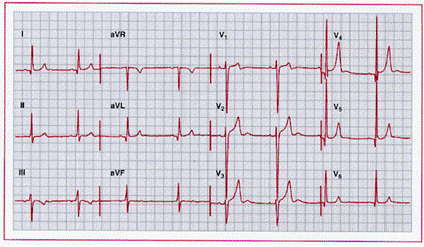
Left ventricular hypertrophy with prominent positive
anterior T waves from a patient with severe aortic regurgitation. This pattern
has been described with “diastolic overload” syndrome but has limited
sensitivity and specificity. Serum potassium was normal.
T WAVE INVERSION
When caused by physiological variants, T wave inversion is sometimes mistaken for ischemia. T waves in the right precordial leads can be slightly inverted, particularly in leads V1 and V2. Some adults show persistence of the juvenile T wave pattern, with more prominent T wave inversion in right precordial to midprecordial leads showing an rS or RS morphology. The other normal variant that may be associated with prominent T wave inversion is the early repolarization pattern. Some subjects with this variant have prominent, biphasic T wave inversion in association with the ST elevation. This pattern, which may simulate the initial stages of an evolving infarct, is most prevalent in young adult black males and among athletes. These functional ST-T changes are probably due to regional disparities in repolarization and can be normalized by exercise.
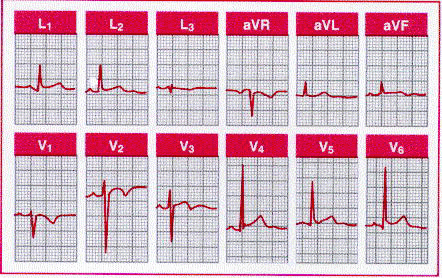
Normal tracing with a juvenile T wave inversion pattern
in leads V1, V2,
and V3, as well as early
repolarization pattern manifested by ST segment elevation in leads I, II, aVf,
V4, V5, and V6.
Primary and Secondary T Wave Inversions
A variety of pathological factors can alter repolarization and cause prominent T wave inversion.As noted above, T wave alterations are usefully classified as primary or secondary. Primary T wave changes are caused by alterations in the duration or morphology of ventricular action potentials in the absence of changes in the activation sequence. Examples include ischemia, drug effects, and metabolic factors. Prominent primary T wave inversion (or in some cases, tall positive T waves) is also a well-described feature of the ECG in cerebrovascular accidents (CVAs), particularly with subarachnoid hemorrhage. The “CVA T wave pattern” is characteristically diffuse, with a widely splayed appearance usually associated with marked QT prolongation. Some studies have implicated structural damage (myocytolysis) in the hearts of patients with such T wave changes, probably induced by excessive sympathetic stimulation mediated via the hypothalamus. A role for concomitant vagal activation in the pathogenesis of such T wave changes, which are usually associated with bradycardia, has also been postulated. Similar T wave changes have been reported after truncal vagotomy, radical neck dissection, and bilateral carotid endarterectomy. In addition, the massive diffuse T wave inversion seen in some patients following Stokes-Adams syncope may be related to a similar neurogenic mechanism. Patients with subarachnoid hemorrhage may also show transient ST elevation, as well as arrhythmias, including torsades de pointes. Ventricular dysfunction may even occur.

Deep T wave inversion can
be due to a variety of causes. Note the marked QT prolongation in conjunction
with the cerebrovascular accident (CVA) T wave pattern caused here by
subarachnoid hemorrhage. Apical hypertrophic cardiomyopathy (HCM) is another
cause of deep T wave inversion that can be mistaken for coronary disease.
In contrast to these primary T wave abnormalities, secondary T wave changes are caused by altered ventricular activation, without changes in action potential characteristics. Examples include bundle branch block, WPW preexcitation, and ventricular ectopic or paced beats. In addition, altered ventricular activation (associated with QRS interval prolongation) can induce persistent T wave changes that appear after normal ventricular depolarization has resumed. The term “cardiac memory T wave changes” has been used in this context to describe repolarization changes subsequent to depolarization changes caused by ventricular pacing, intermittent LBBB, intermittent WPW preexcitation, and other alterations of ventricular activation. Finally, the designation idiopathic global T wave inversion has been applied in cases in which no identifiable cause for often marked, diffuse repolarization abnormalities can be defined.