# Properties of Pure
substance:
Pure substance: these have homogenous and invariable chemical
composition. It may exist in more than one phase, but chemical composition
is same in all phases.
Air – exception as pure substance
(why?)
Phase: quantity of matter that is homogenous throughout. (forms in
which pure substance can exist).
Gibbs phase rule: gives the no of independent properties required
to fix a state.
f
= C-P +2
f: no of
independent properties
P: no of phases
C: no of pure
substances
# Vapor- liquid
– solid phase equilibrium in pure substance
Phase boundary
is the interface where transition of two phases occurs.
Phase
boundaries can be represented in P-T diagram.

#
P-V and T-V diagram of water.

Define:
Ø
Saturated
liquid line
Ø
Saturated
vapor line
Ø
Specific
volume for two phase line
Ø
Super
heated vapor
#
Quality:
a property valid for only two phase line, defined as
for
system volume V, and having liquid volume Vl and gaseous volume
Vg and total mass m, we have the following relation:

This relation is true for all extensive properties
of the pure substance.
#
Liquid – Vapor phase diagram:
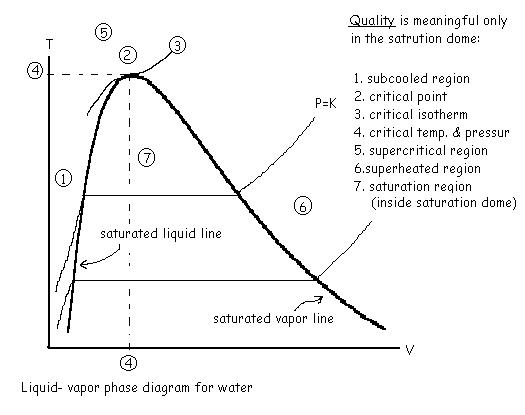
Steam Table:
It is a table in which different values of various properties of
steam within and beyond saturation region are listed. Values are listed
there in different tables: 1. According to different saturation
temperature (temperature table), 2. According to different saturation
pressure (pressure table) and 3. Values in the super heated region
(superheated table).
# Equation of state:
It is a mathematical function relating the appropriate
thermodynamic co-ordinates of a system in equilibrium. For a closed system
equation of state relates temperature to two other thermodynamic
variables.
It can be established either by molecular theory or by experiment.
# Equation of state for Ideal gas:
Fundamental
property of gas is that
![]() is independent of nature of
gas and depends only on temperature T (experimentally found out).
is independent of nature of
gas and depends only on temperature T (experimentally found out).
It can be deduced that for any gas,
![]()
Where,
![]() is the molecular volume and
is the molecular volume and
![]() is the Universal gas constant.
is the Universal gas constant.
Ideal
gas is the gas, which obeys
![]() .
.
In this
equation as
![]() (i.e. –273.15oC)
(i.e. –273.15oC)
![]() ; Keeping volume constant
; Keeping volume constant
![]() ; Keeping pressure constant
; Keeping pressure constant
Since,
P and v can’t be negative, lowest possible temperature is 0 K.
Now,
we have, if V is the total mass, and n is no. of moles; and m is
molecular weight of the gas.

And R is called characteristic gas constant.
Now
at P=101.3 MPa; T=273.15 K; V=22.4 m3

For
O2, R=83143/32 = 0.262 KJ/Kg K
And
for air, R=8.3143/28.96 = 0. 287 KJ/Kg K
At
low densities gases obey Charl’s and Boyle’s laws; concluded by
experimental observations.
The
assumptions of ideal gas are