Study Card Boxes: Box 1 Box 2 Box 3 Box 4 Box 5 Box 6 Box 7 Box 8 Box 9 Box 10
Day 1: Graphing
Steps for making a graph.
Types of Graphs:
Circle- Compare how one part relates to the whole.
Bar- Compare measurements or quanties.
Line- Compare two or more sets of data. Showing a trend or pattern. Also showing a change over time.


Day 2: States of Matter
What is matter? Matter is anything that has mass and volume. All matter is made up of atoms or particles. These atoms/particles all take up certain amounts of space. Mass and volume are the two properties that all states of matter share. In addition each state of matter has its' own properties.
They are listed below:
Slow Moving
Low Energy
Definite Shape
Definite Volume
Medium Energy
No Definite Shape
Definite Volume
High Energy
No Definite Shape
No Definite Volume
Day 3: Relating Mass and Volume
We discussed th fact that all matter shares two of the same properties: mass and volume. Mass is the number of particles an object contains. Mass and weight should not be confused. Weight is measures the effects of gravity. The moon has less gravity than earth. If you traveled to the moon your mass would stay the same but your weight would change due to the effects of gravity.
All object are made up of particles or atoms. These particles all take up space, which is the objects' volume. Mass and volume are directly related. This relationship can be described as the objects DENSITY. There is a certain ratio of the number of particles and the space they take up. Each piece of matter has its own density. Try this experiment to see if you can come up with the relationship between the mass and volume of water.
Box 4:Density
Density is the relationship between mass and volume. This can be stated simply: Density is how much stuff you have compared to how much space it takes up. For us here on Long Island we can view density by looking at New York City. NYC is filled with MANY people. NYC is more densely packed than Long Island. Especially out east by all of the farms. The formula for density is mass divided by volume. This formula can be placed in the triagle below.
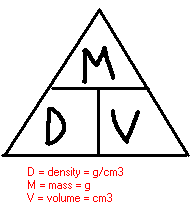
Did you miss a homework ditto on 10/5? Click here
Box 5:Density2
Ths density of an object is the relationship between mass and volume. Take a marshmellow for example, it has a certain density. As soon as we squish it we are moving the molecules into a smaller space. Therefore changing its density. By taking the same amount of particles and moving them into a smaller space we made the marshmellow increase its density. What if we took an object and cut it in half? Would that change the density? In this case the density would remain the same. Try this little problem: What is the density of an object with a mass of 24g and a vloume of 4cm3? Using the above formula we can say that the density of this object is 6g/cm3. Now lets cut the mass and the volume in half. What is the density of an object with a mass of 12g and a volume of 2cm3? Now using the same formula we have a density of 6g/cm3. Both densities are the same even though we cut the object in half. Think back to the lab we did in class: Relating Mass and Volume. We calulated the density of water for each trial. Did the density of water change at each trial? No the density of water always remained 1g/cm3.
Box 6:Finding Unknowns
All elements have their own identifying densities. For example: Gold=19.3g/cm3, Aluminum= 2.7g/cm3 and Hygrogen 0.0009g/cm3. By calculating the density of an object a scientist can figure out the identity of an unknown substance. We will be doing this lab in class to figure out the name of some unknowns.
Box7: Density Lab
In this box you will describe the density lab we completed in class.
This page is still being built. Check back by October 11th for the rest of the notes and class activities.
Thanks