There is a point on the Kelvin scale where there is absloutely NO MOTION of the particles (0 degrees Kelvin). The point is called Absolute Zero. At absolute zero the motion of the particles stop. this is the coldest temperature possible.
The three states of matter are as follows: Solid, Liquid and Gas. Each state has different amounts of kinetic energy (energy of motion). Use the chart below to help out. Conduction is the method of heat transfer by collision of particles. This method usually occurs in solids. Have you ever tired to open a door in the summer time and the door sticks??? Have you ever seen cracks in the side walk and wonder how they got there??? When heat is added to an object the particles move around. As the particles move around they need more space or expand. Expansion means to get larger. Objects expand when heat is added because the particles move faster. The opposite is true when heat is removed. Objects contract, get smaller, when heat is removed because the particles slow down and take up less room. So during the summer time the doors in your home expand due to the increase in heat. They get too big for the opening, so the door gets stuck. The same is true for the cracks in the sidewalk. If you have ever looked at the sidewalk you will notice gaps or rubber spaces inbetween each segment of the sidewalk. These gaps or rubber spaces are to help absorb some of the expansion of the sidewalk.
A thermometer is used to measure temperature. Temperature is the average kinetic energy and is measured in units called degrees. Not to be confised with heat, total kinetic energy, which is measured in calories. There are three types of thermometers:
Study Card Boxes: Box 1 Box 2 Box 3 Box 4 Box 5 Box 6 Box 7 Box 8 Box 9 Box 10
Box 1: Heat Energy
You have experienced heat at some point in you life. Heat makes molecules move faster. Heat is total the kinetic energy of a substance. Kinetic energy is the energy of motion. (refresher on kinetic and potential energy) The kinetic theory of heat states that as heat is added to a molecule the particles within that molecule move faster. Temperature is the average kinetic energy of a substance. For example a match has a high temperature, much higher than that of a block of ice. However, the total heat energy of these two items is much different. Since the block of ice has more moving particles than the match, the block of ice has a higher total kinetic energy, therefore higher heat energy.
Box 2: Temperature Scales
The following is a chart of the three temperature scales: Celsius, Fahrenheit and Kelvin.
of
Water
of
Water
Other Countries
Box 3: States of Matter
Have you ever taken a shower and when you are done the mirror is all foggy? How about beads of water forming on your glass during the humid days of summer? these are both examples of phase changes due to the subtraction of heat. You have hot steam escaping from your shower. Only to land on a cold mirror. This cold mirror takes the heat from the steam and turns it into water. The same concept is true for the droplets of water that form on your glass in the summer time. the water vapor in the air comes in contact with your ice cold glass. The glass take the heat away from the water vapor in the air turning the vapor into little droplets of water.
Slow Moving
Medium Speed
High Energy
Try to think of a liquid as water, a solid as ice and a gas as steam. How do you change ice into water? You need to add heat energy. Look at the diagram above. Liquids have more energy than solids. The diagram below will help to explain some more of the vocabulary words for this section. I would recommend that you draw this diagram into your study card.
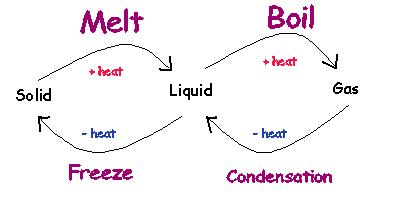
By following the diagram you can get the definitions for the following words:
Melt- The process of changing a solid to a liquid by the addition of heat.
Boil- The process of changing a liquid to a gas by the addition of heat.
Freeze- The process of changing a liquid to a solid by the subtraction of heat.
Condensation- The process of changing a gas to a liquid by the subtraction of heat.
Now for one more set of voaculary words. i am going to tell you to use the same diagram of solids, liquids and gases. Except this time remember that the word point means "temperature at which."
Melting point- The temperature at which a solid turns into a liquid.
Boiling point- The temperature at which a liquid turns into a gas.
Freezing point- The temperature at which a liquid turns into a solid.
Condensation point- The temperature at which a gas turns into a liquid.
Box 4:Conduction
Box 5: Convection
Convection is the method of heat transfer in liquids or gases by means of currents. As particles get heated they start to move faster. As the particles move faster the object becomes less dense. Less dense object move to the surface of more dense objects. that explains why the warmer water when swimming is at the top. This also explains how a hot air ballon works. Most likely your home is heated by convection. A furnace heats up water. At this water heats up the particles move faster. The heated particles cirulate throughout the house while the cooler particles move in and take there place.
Box 6: Radiation
Radiation is the method of heat transfer though empty space. Heat is radiated from the sun to earth through outer space. Heat radiation also comes from a fire place. Heat that comes out from the sides also provides us with infrared or heat radiation. How do you think your bread gets toasted???? Could the answer be heat radiation????
Box 7: Conductors and Insulators
A conductor allows the flow of heat. Usually meatls make some of the best heat conductors. Aluminum and copper are used in pots and pans because of their heat conduction properties. Stainless Steel does not conduct heat as well as copper or aluminum. Therefore, we use stainless steel as our flatware.
An insulator blocks the flow of heat. We have insulation in our homes to keep the heat in during the winter and keep the heat out during the summer. Wood, glass, plastic, and down are all examples of insulators. When cooking at home why do you think it is a good idea to use a wooden spoon over a metal one?
Box 8: Thermal Expansion
Box 9: Thermometers
Alcohol- red in color- great in low temperatures
Mercury- silver in color- liquid metal- great in high temperatures.
Bi-Metal- 2 metal strips bonded together. The metals expand at different rates causing the strip to curl.