
Study Card Boxes: Box 1 Box 2 Box 3 Box 4 Box 5 Box 6 Box 7 Box 8
Quick Reference Element/Symbol
We have spent much of our time talking about particles that are all around us. We spoke of “atoms”. How everything is made up of these atoms. Another name for the atom is an element. An element is the building block of matter. An element is the smallest unit of matter than cannot be broken down any further. Elements can either be monatomic (one atom) or diatomic (two atoms). Diatomic atoms are two atoms that are joined together naturally in nature. If two or more elements combine we then have what is called a compound. Compounds can be broken down by chemical means. Some common compounds are water (H2O), sugar (C We can now take these compounds and combine them together to make a mixture. A mixture is a physical combination of two or more kinds of matter (compounds). Mixtures can be separated by physical means such as; heating or cooling, removing with your hand, using a filter and paper chromatography. There are two types of mixtures, homogeneous and heterogeneous. A homogeneous mixture looks the same through out. Take chocolate milk, it is a mixture of milk and chocolate. You can no longer tell which parts are milk or chocolate. After a few hours of sitting undisturbed you surely will be able to see all the chocolate sitting on the bottom of the glass. Aheterogeneous mixture still shows signs of each of the parts. Fruit salad is a great example. You have a mixture of fruit yet you can still make out each individual piece. Protons and neutrons are found in the nucleus of the atom or element. The electrons are found on the outside of the nucleus spinning in orbitals called energy levels. The first energy level contains only two electrons. The second and third energy levels contain eight electrons each (We will not be diagramming any elements larger than that.). Below are some diagrams of different elements. Ionic Compounds is a combination of positive and negative ions. An ion is either a positively or negatively charged "atom". An atom becomes an ion when it either gains or looses electrons. Ions are usually a three dimensional crystal structure when dissolved in water conduct electricity. For example, salt is an ionic compound (see page 473 in your text book for a diagram). Sodium (group 1) has one electron in its outer shell, while Chlorine (group 17) has 7 electrons in its outer shell. One electron leaves sodium and jumps to chlorine to complete the outer shell. This jump leaves sodium with a positive charge and chlorine with a negative charge. Once salt is disolved in water, salt water becomes a great conductor of electricity. Hey, didn't you learn never to go swimming at the beach during a thunder storm? Try a few practice problems:
What do you think salt water is? (element, compound, heterogeneous or homogeneous mixture)
You agenda has a periodic table of the elements in the front science section.
reflect light when polished
luster
silver-gray in color
except gold/copper
solids at room temperature
except mercury
good conductors of electricity and heat
malleable
except Al
Semi-conductors
no single color
brittle
poor conductors of electricity and heat
Neon
Argon
Xenon
Krypton
Radon
Do Not combine with other elements

Atomic Weight: Tells you the number of protons and neutrons. Zinc has 65.39 protons and neutrons in its nucleus.
By having this information we can figure out how many electron, protons and neutrons are in each atom or element. Lets look at Zinc’s atomic number. Zinc has 30 protons. Elements in there neutral state have equal number of protons and electrons. So if Zinc has 30 protons then it also has 30 electrons. Now that we know the number of protons and electrons lets figure out the neutrons. The atomic weight (mass) of Zinc is 65.39. Since this number represents both protons and neutrons we can subtract the number of protons we have already calculated (65.39 – 30 = 35.39). Zinc has 35.39 neutrons.


As you can see some of the atoms you had drawn had some empty spaces in their outer energy levels. Take a look at the atoms in group one of the periodic table. They all have one electron in their outer shell. All the electrons in group 2 have 2 electrons in their outer shell. The noble gases have their last shell or energy level filled with electrons so they really do not combine with other elements.
A Moleculer Compound is a compound made up of molecules. Molecules usually share electrons (covalent bonds)in thier outer shells. For example a molecule water is made up of 1 oxygen and 2 hydrogen atoms. Oxygen needs two electrons to fill its outer shell so it shares an electron with hydrogen.

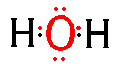 We can show this sharing of electrons in the form of an electron dot diagram. One dot for each electron in the outer shell.
We can show this sharing of electrons in the form of an electron dot diagram. One dot for each electron in the outer shell.
back to top
Compounds are combinations of different elements. For example, H2O is water. Water contains 2 atoms of Hydrogen and 1 atom of Oxygen. The subscript next to the letter represents how many of that atom is present in the compound. If there is no subscript then there is one 1 of that atom. Lets try a few more.
1Chlorine
8 Hydrogen
4 Oxygen
(sugar)
22 Hydrogen
11 Oxygen
Chemical reactions occur when an object has made a chemical change. Chemical changes happen when you mix ingredients together to bake a cake or make brownies. When a chemical reaction takes place bonds break and new bonds and product are formed. Heat is always involved in a chemical reaction. Heat is either added or taken away in the form of energy. Below are some samples of a chemical formula which signify a chemical reaction.

The number of elements on one side of the equation is ALWAYS equal to the number of element on the other side of the equation.
Try balancing these equations:
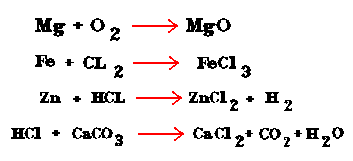
(once you are done click to see the answers.)
Exercise 1
Exercise 2
A physical change is a change in appearance only. Usually a phase change like water turning into steam. That is a physical change. Any mixture can be a physical change. The objects that are being combined still retain properties before and after the combination. Physical changes can result from filtering, freezing, melting, condensation, boiling, evaporation, tearing, and crushing to name a few.
During a chemical change, substances react and combine in ways to form new substances with different physical and chemical properties. For example: burning of wood, cooking an egg, rusting of iron, and souring of milk.
These elements and symbols can be placed in boxes 9 and 10 if you would like.