Molecular BIology of Cancer Topics
![]()
![]()
Neu Receptor Tyr Kinase
The Neu oncogene (aka erbB2, HER2) encodes a receptor tyrosne kinase similar to the epidermal growth factor receptor. In the rat, the oncogene has a missense mutation: Glu (GAG) substitutes Val (GTG). As a result of the Neu missense mutation, receptor aggregation occurs in absence of ligand. Oncogne Neu is thus constitutively activated and will increase the activity of proteins activated by Neu, for example phospholipase Cg., and induce the malignant transformation of cells.
|
DHFR/G8 cells (wild-type Neu) and B104 cells (Neu oncogene) were treated or not with a cross-linking agent that covalently binds proteins together. Neu protein was immunoprecipitated and resolved by SDS-PAGE. High molecular weight Neu dimers were detected in the wild type DHFR/G98 cells only when treated with the cross-linking agent. High molecular weight Neu dimers were detected in the oncogene B104 cells with and without crosslinking agent treatment. (Nature 330:230-231, 1989)
|
Wild-type Neu is amplified in 28% of human breast cancer and 27% of human ovarian cancer patients, and the number of copies of the gene correlates inversly with survival time.
Herceptin (rhuMAb, HER2) is an antibody against Neu that inhibits Neu activity. According to Baselga et. al., recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/Neu overexpressing human breast cancer xenografts (Cancer Res. 58: 2825-2831, 1998).

In a Phase II study of weekly
intravenous Herceptin in patients with HER2/Neu-overexpressing metastatic breast
cancer, therapy caused tumor regression or stable disease in many of the treated
patients.Without Herceptin, all patients probably would have had disease progression.
(Baselga et al. J. Clin. Oncol. 14: 737-744, 1996)
 Non-receptor
Tyrosine Kinases
Non-receptor
Tyrosine Kinases
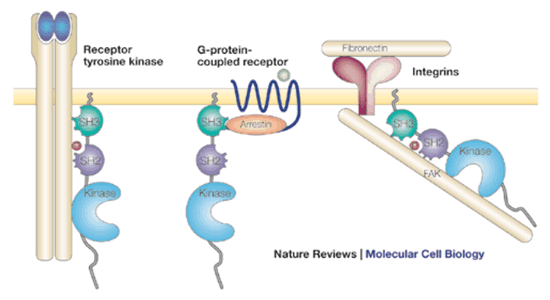
The Src family of non-receptor tyrosine kinases include Src, Yes, Fgr, Lck, Fyn, Lyn, and Hck. They associate with inner face of plasma membrane through a myristic acid lipid tail and contain SH3 and/or SH2 domains, a tyrosine kinase domain and tyrosines available for phosphorylation.
Src is regulated by phosphorylation of at least two tyrosines. Phosphorylation of Tyr 416 increases Src activity, while phosphorlation of Tyr527 decreases activity.

Src can be activated differently by the diverse signaling pathways in which it participates. Its SH2 domain binds phosphotyrosine of activated growth factor receptor/tyrosine kinase, then Src can be phosphorylated by the receptor tyrosine kinase. Alternatively, SH3 can bind other proteins associated with G-protein coupled receptors. Adaptor proteins such as GRB2 bind Src phosphotyrosines.

 When
Tyr 527 is phosphorylated, Src’s own SH2 domain binds to it, and the Src
kinase domain is in the repressed, inactive conformation. When Tyr 527 is unphosphorylated,
the Src tyrosine kinase domain is in the open, activated conformation and Tyr
416 can be phosphorylated to help activate Src.
When
Tyr 527 is phosphorylated, Src’s own SH2 domain binds to it, and the Src
kinase domain is in the repressed, inactive conformation. When Tyr 527 is unphosphorylated,
the Src tyrosine kinase domain is in the open, activated conformation and Tyr
416 can be phosphorylated to help activate Src.
Experimental results demonstrate the importance of Tyr 527 in Src regulation:
 In
addition to the C-terminal deletion (Tyr 527 is missing) that makes it oncogenic,
the viral form of src (v-src) has several amino acid substitutions due to point
mutations in the v-src oncogene.
In
addition to the C-terminal deletion (Tyr 527 is missing) that makes it oncogenic,
the viral form of src (v-src) has several amino acid substitutions due to point
mutations in the v-src oncogene.
Another oncogenic form of non-receptor tyrosine kinase is coded by the Philadelphia chromosome. Chromosomal translocation between bcr and c-abl genes create a fusion gene and thus a fusion protein. The translocation is t(9;22)(p34;q11). The c-abl proto-oncogene encodes a nonreceptor tyrosine kinase related to Src. Human patients with chronic myelogenous leukemia (CML), acute lymphocytic leukemia (ALL), and acute myelogenous leukemia (AML) exhibit the Philadelphia chromosome in the leukemic cells. Virtually all CML patients have the bcr-abl oncogene.
 The
bcr promoter sequences are required for bcr-abl -induced transformation
to occur. In the fusion protein, the Bcr domain binds the SH2 domain of Abl,
therefore Abl cannot bind its own phosphotyrosine (like Src Tyr 527) to shut
itself off and remains constitutively active.
The
bcr promoter sequences are required for bcr-abl -induced transformation
to occur. In the fusion protein, the Bcr domain binds the SH2 domain of Abl,
therefore Abl cannot bind its own phosphotyrosine (like Src Tyr 527) to shut
itself off and remains constitutively active.
|
Radiolabeled lysates of Sf9 cells infected with wild-type baculovirus or P160 Bcr recombinant baculovirus were incubated with GST, GST-SH3, GST-SH2, GST-SH3/SH2 or anti-Bcr antibody. Afterwards bound proteins were resolved by SDS-PAGE. Bcr was detected bound to SH2 or anti-Bcr only in the Bcr recombinant lysates. (sorry I lost the reference)
|
![]()
![]() Continue to "Ras" or take a quiz: [Q1].
Continue to "Ras" or take a quiz: [Q1].
Need more practice? Answer the review questions below.
Questions under construction
Continue scrolling to answers below.
Hey! DON'T PEEK!!! Finish the questions fist!
Answers under construction