Cellular and Molecular Biology Topics
![]()
![]()
Signaling
Cells communicate with each other by either close contact or by secreted signaling molecules. Gap junctions allow direct communication between the cytoplasms of adjacent cells. Adjacent cells may also communicate by cell surface ligand/receptor interactions (juxtacrine). Neurotransmitter release at a synapse relates a message from one cell to the next. Other extracellular signaling molecules may act on the same cell that secreted them (autocrine) and adjacent cells (paracrine), or on a distant target by being transported in the circulation (endocrine).

Signaling molecules may be peptides, proteins, steroids, amino acids or other. Examples of signaling molecules includes hormones like insulin, growth factors like platelet-derived growth factor (PDGF), cytokines like interleukin, neurotransmitters like acetylcholine, ecosanoids like prostaglandin or gases like nitrous oxide (NO).
The first step in a signaling pathway is the activation of a receptor by an extracellular signaling molecule. The activated receptor then will either directly influence a response or induce the generation of a second messenger that carries the signal into the cytoplasm, affecting the regulation of another target molecules or otherwise influencing a response. The response may be a control of function, like secretion, metabolism or contraction, or a control of fate, like cell growth/division, differentiation or apoptosis.
 Each
cell has its own complement of receptors. Cells can receive multiple signals
simultaneously and they will be integrated. The same extracellular signals can
affect different cells differently, and the same intracellular signal can cause
different effects in different cells. A limited number of receptors-signal paradigms
connect receptors to target. Most signals provoke their own down-regulation
(feedback).
Each
cell has its own complement of receptors. Cells can receive multiple signals
simultaneously and they will be integrated. The same extracellular signals can
affect different cells differently, and the same intracellular signal can cause
different effects in different cells. A limited number of receptors-signal paradigms
connect receptors to target. Most signals provoke their own down-regulation
(feedback).
Common second messengers include cyclic nucleotides, phospholipid-derived molecules, and gases like NO. The cyclic nucleotides are cyclic AMP (cAMP) and cyclic GMP (cGMP). Phospholipid-derived second messengers include the phosphatidylinositol (PIP3) derivatives diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), and other molecules like ceramide.
 Cyclic
nucleotides are produced from either ATP or GTP by cyclases (adenylyl cyclase
and guanylyl cyclase, respectively) and degraded by phosphodiesterases. Cyclic
nucleotides may be generated by membrane-bound adenylyl cyclase after activation
by a G-protein, by receptor guanylyl cyclase activated by extracellular messengers
or by soluble guanylyl cyclase activated by NO. Receptor guanylyl cyclase has
a pseudo kinase domain of unknown function, which may just bind ATP. They diffuse
in the cytoplasm and activate other effector proteins, like the cAMP-dependent
protein kinase (PKA) and cGMP-dependent protein kinase (PKG).
Cyclic
nucleotides are produced from either ATP or GTP by cyclases (adenylyl cyclase
and guanylyl cyclase, respectively) and degraded by phosphodiesterases. Cyclic
nucleotides may be generated by membrane-bound adenylyl cyclase after activation
by a G-protein, by receptor guanylyl cyclase activated by extracellular messengers
or by soluble guanylyl cyclase activated by NO. Receptor guanylyl cyclase has
a pseudo kinase domain of unknown function, which may just bind ATP. They diffuse
in the cytoplasm and activate other effector proteins, like the cAMP-dependent
protein kinase (PKA) and cGMP-dependent protein kinase (PKG).
The PIP3 derivatives are created by phospholipase C (PLC) and act by opening Ca2+ channels in the plasma and ER membranes. This in turn activates protein kinase C (PKC), which needs both binding to DAG and to CA2+ to be activated.
Nitric oxide is an endothelium-derived muscle relaxing factor generated as needed by nitric oxide synthase (NOS) from arginine. The constitutive endothelial NOS, can be activated in response to an increase in Ca2+ concentration following stimulation with receptor-dependent agonists such as acetylcholine and bradykinin.

![]() NO +
NO + 
arginine
citrulline
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
G-Proteins
There are hundreds of G-protein coupled receptors, including receptors for hormones, neurotransmitters, photons, taste and olfaction. They have a transmembrane domain containing seven alpha helices, with linker sequences glycosylated in extracellular regions and the intracellular carboxy terminus available for phosphorylation.
The G-protein itself has an abg structure, with the a subunit having intrinsic GTPase activity. Although a is generally considered the main signaling subunit, bg are also involved in signaling.
 The
classic example of a G-protein coupled receptor is the b-adrenergic
receptor. Ligand bound to the receptor promotes a conformational change of the
G-protein and exchange of the GDP bound to a
for GTP. a
the dissociates from bg
and activates adenylyl cyclase. The GTPase activity of a
quickly hydrolyzes GTP to GDP and a
returns to
form a trimer with bg.
The
classic example of a G-protein coupled receptor is the b-adrenergic
receptor. Ligand bound to the receptor promotes a conformational change of the
G-protein and exchange of the GDP bound to a
for GTP. a
the dissociates from bg
and activates adenylyl cyclase. The GTPase activity of a
quickly hydrolyzes GTP to GDP and a
returns to
form a trimer with bg.
This mechanism is desensitized by bARK phosphorylation of the receptor. Phosphorylated receptor cannot bind GDP-, therefore signaling is disabled. Another way to phosphorylate the receptor is by PKA, thus cAMP acts as a feedback inhibitor of itself.
The b-adrenergic receptor Gs protein activates adenylyl cyclase, but other G-proteins have different effects. The a2-adrenergic receptor Gi protein inhibits adenylyl cyclase. The Gq protein of the a1-adrenergic receptor activates phospholipase C-b. Transducin, the Gt protein of the rhodopsin receptor activates cGMP phosphodiesterase.
 Both
trimeric G-proteins and small GTPases need GTP to change into their active conformastion.
Although they both have intrinsic GTPase activity, small GTPases need to bind
a GTPase activation protein (GAP) to activate their GTPase activity. A guanine
nucleotide exchange factor (GNEF) is needed by small GTPases to exhange GDP
for GTP.
Both
trimeric G-proteins and small GTPases need GTP to change into their active conformastion.
Although they both have intrinsic GTPase activity, small GTPases need to bind
a GTPase activation protein (GAP) to activate their GTPase activity. A guanine
nucleotide exchange factor (GNEF) is needed by small GTPases to exhange GDP
for GTP.
Ras is a small GTPase associated with cell proliferation, found in more than 30% of tumors. It is activated by growth factor receptors. After dimerization and cross-phosphorylation of tyrosine kinase domains in the receptor, the SH2 domain of Grb2 binds to the receptor and Sos binds to the SH3 domain of Grb2.

Sos promotes dissociation of GDP form Ras (i.e. Sos is a GNEF). After GTP binds and activates Ras, Sos dissociates. Ras can then signal through the MAP kinase pathway to modulate gene expression. Ras activates MAPK by phosphorylating it. Phospho-MAPK forms dimmers that translocates to the nucleus and catalyze the activation of transcription factors.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Protein Phosphorylation
Protein kinases and phosphatases regulate other proteins by adding or removing a phosphate group. Kinases use ATP to add a phosphate at a hydroxyl side chain. Phosphatases catalyze the hydrolysis of a phosphate side chain to yield a hydroxyl. In general, phosphorylated proteins are inactive, although some proteins are activated by phosphorylation (?).
Kinases and phosphatases may be specific for Ser/Thr or for Tyr, or may have dual specificity (Ser/Thr/Tyr) They may be cytoplasmic or associated to membranes. Some examples of Ser/Thr kinases are PKA, PKG, Ca2+ activated kinases (PKC), and cycling-dependent kinases. Examples of Tyr kinases are the src family and various receptor Tyr kinases. MAP/ERK is a dual specificity kinase. Ser/Thr phosphatases include PP1, PP2A, PP2B (calcineurin) and PP2C. Tyr phosphatases include CD45 and PTP1B. PTEN is a lipid kinase (?).
Protein kinases are regulated by either phosphorylation, allosteric effector, ligand binding, synthesis/degradation equilibrium and targeting to the right place at the right time. An example of allosteric regulation is PKA, which is a tetramer of two regulatory and two catalytic subunits. When cAMP binds the regulatory subunits, the catalytic subunits are release from the tetramer and are available to phosphorylate other proteins.
Tyrosine phosphorylation
is elevated in transformed cells (i.e. cancer). In normal cells only 1/2000
proteins is phosphorylated. The transformed gene of Rous sarcoma virus (src)
encodes a tyrosine kinase (pp60). Hormones and growth factors bind receptor
tyrosine kinases, for example endothelial growth factor (EGF) and insulin receptors.
These receptors are activated by dimerization and cross-phosphorylation of each
subunit. The active receptors can bind to and activate SH2 containing proteins.
Some, like the insulin receptor, also phosphorylate other proteins that can
interact with SH2 containing proteins.
 The
first example of protein-protein interactions in cellular signaling was the
SH2 domain, which is ~ 100 amino acids long. Similar versions of SH2 occur in
many proteins, for example PLC-g,
Grb2, GAP, and p85. SH2 binds phosphotyrosine-containing sequences in other
proteins. Kinases bind to SH2 and phosphorylate a region outside SH2 to activate
a protein. Another common domain, SH3, binds to proline-rich sequences. The
adaptor protein Grb2, which plays a role in Ras signaling, contains both SH2
and SH3 domains.
The
first example of protein-protein interactions in cellular signaling was the
SH2 domain, which is ~ 100 amino acids long. Similar versions of SH2 occur in
many proteins, for example PLC-g,
Grb2, GAP, and p85. SH2 binds phosphotyrosine-containing sequences in other
proteins. Kinases bind to SH2 and phosphorylate a region outside SH2 to activate
a protein. Another common domain, SH3, binds to proline-rich sequences. The
adaptor protein Grb2, which plays a role in Ras signaling, contains both SH2
and SH3 domains.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Signaling Pathway
Phospholipase C (PLC) cleaves the membrane lipid phosphatidylinositol 4,5 phosphate (PIP2) into 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3).

DAG stays in the membrane and can interat there with PKC, localizing the kinase to the membrane. IP3 diffuses to the ER and activates Ca2+ channels. The released calcium binds PKC and activates it.

Rhodopsin is a G-coupled receptor that absorbs one photon to activate its G-protein known as transducin. Transducin then activates a phosphodiesterase that hydrolyzes cGMP. The lowered cGMP concentration leads to closure of Na+ channels and hyperpolarization of the cell.
Acetylcholine receptors in endothelial cells activate calmodulin, a small ubiquitous Ca2+ binding protein that confers Ca2+ control of many enzyme, such as protein kinases and NO synthase (NOS). Activation of NOS in endothelial cells produce NO that diffuses to adjacent smooth muscle and activates soluble guanylyl cyclase to generate cGMP.
When T-cell receptors are activated by a presenting cell, the tyrosine kinase activity of the receptor activates PLC-gamma, which in turn increases Ca2+ concentration by activating DAG and IP3. Ca2+ then cativates calcineurin, which in turn activates the transcription factor for interleukin 2 (NF-AT). Interleukin 2 is an autocrine signal for proliferation (IL2 gene). Cyclosporin inhibits calcineurin, thus preventing T-cell proliferation.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
The Hypothalamic-Pituitary-Adrenal Axis
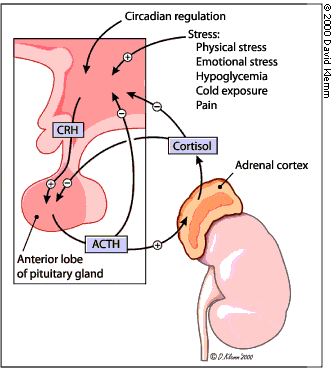 Adrenocorticotropic
hormone (ACTH) is derived from the proteolitic processing of pro-opiomelanocortin
(POMC). Other products from POMC are b-endorphin g-MSH and g-LPH.
Adrenocorticotropic
hormone (ACTH) is derived from the proteolitic processing of pro-opiomelanocortin
(POMC). Other products from POMC are b-endorphin g-MSH and g-LPH.

ACTH is released by the pituitary in response to corticotropin releasing hormone (CRH) from the hypothalamus. Cortisol acts as a feedback inhibitor of ACTH release.
ACTH acts on a membrane surface receptor in the adrenal cortex. The adrenal ACTH receptor is a G-protein coupled receptor that increases the intracellular concentration of cAMP. IN turn, PKA activity increases, which activates cholesteryl ester hydrolase. This enzyme catalyzes the conversion of stored cholesteryl esters to cholesterol, making them available for steroid synthesis.
Steroids can pass through plasma membranes and bind transcription factors which in turn interact with cis-acting sequences of the genome. Each steroid may have differential effects on different cells.
Nuclear receptors have important roles in development, metabolism and reproduction. They mediate transcriptional response in target cells to sex steroid (progestins, estrogens, amdrogens), adrenal steroids (glucocorticoids and mineralocorticoids), vitamin D3, thyroid hormone, retinoids and metabolic ligands. These ligands are small, fairly rigid molecules with a high degreee of hydrophobicity that facilitates their entry into cells by simple diffusion.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Steroid Hormone Synthesis
Either cytochrome P450 Side Chain Cleavage (P450 SCC) enzyme or 11-b-hydroxylase (?) catalyze the cleavage of the aliphatic side chain in cholesterol, yielding the precursor to all other steroids, pregnenolone.

In this reaction, electrons are donated from NADPH to adrenodoxin reductase, which reduces adrenodoxin. Adrenodoxin serves as the reductant of the mitochondrial P450 SCC.
Variuos reactions then catalyze the transformation of pregnenolone to either mineralocorticoids, glucocorticoids or sex hormones. In summary, 3-b-hydroxysteroid dehydrogenase generates progesterone from pregnenolone. Progeterone is then converted to mineralocorticoid precursors by 21-hydroxylase, and to glucocorticoid and sex hormone precursors by 17-a-hydroxylase. 21-hydroxilase and 11-b-hydroxylase are responsible for the synthesis of cortisol from intermediate precursors.

Congenital adrenal hyperplasia occurs when there is a deficiency in one of the hydrolases involved in steroid synthesis. 3-b-hydroxysteroid dehydrogenase deficiency prevents synthesis of glucocorticoids, mineralocorticoids and sex hormones. Patients suffer a marked salt excretion and die early.


17-a-hydroxylase deficiency prevents the production of sex hormones and cortisol, and increases the synthesis of mineralocorticoids. This causes sodium and fluid retention, leading to hypertension. Patients are phenotypically female but are unable to mature.
The most common deficiency, 21-hydroxylase, is usually partial. ACTH levels are elevated, sex hormone levels are elevated and there is masculinization. Reduced mineralocorticoid levels leads to sodium loss, dehydration and hypotension. In this case, cortisol is given to decrease ACTH levels.

11-b-hydroxylase deficiency leads to decreased cortisol and aldosterone levels. Increased deoxycortisone levels cause fluid retention, hypertension and masculinization.

Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Nuclear Hormone Receptors
 The
receptors of the nuclear hormone superfamily have three domains: ligand-binding
domain, DNA-binding domain and a transactivation domain of variable length and
sequencethat binds with other proteins associated with the promoter region of
the gene. The DNA binding domains share similar amino acid sequences that form
zinc fingers. Members of the nuclear hormone superfamily bind steroids like
estrogen (ER), progesterone (PR) and glucocorticoids (GR), and peptides like
thyroid hormone (TR), retinoic acid (RAR), vitamin D (VDR) and peroxisome proliferators
(PPAR). Orphan receptors are members of the nuclear receptor family whose regulatory
ligands are as yet unknown.
The
receptors of the nuclear hormone superfamily have three domains: ligand-binding
domain, DNA-binding domain and a transactivation domain of variable length and
sequencethat binds with other proteins associated with the promoter region of
the gene. The DNA binding domains share similar amino acid sequences that form
zinc fingers. Members of the nuclear hormone superfamily bind steroids like
estrogen (ER), progesterone (PR) and glucocorticoids (GR), and peptides like
thyroid hormone (TR), retinoic acid (RAR), vitamin D (VDR) and peroxisome proliferators
(PPAR). Orphan receptors are members of the nuclear receptor family whose regulatory
ligands are as yet unknown.
Nuclear receptor agonist are ligands that bind to a nuclear hormone receptor and enhance their function. For example, estradiol binds the estrogen receptor (ER) and induces female reproductive functions. Antagonists are ligands that prevent agonist association with the receptor but do not induce any receptor action. For example tamoxifen, an important breast cancer treatment, binds ER and inhibits its function. RU 486 is an antagonist of the progesterone receptor used to induce abortion. Antagonists generally have a similar skeleton as the corresponding agonist plus a large extension from the approximate center of the molecule.
Selective ER modulators (SERM) are ligands that in some tissues act like estrogens, but block estrogen action in others tissues. Tamoxifen is a SERM in that it inhibits ER in breast but functions as an agonist in bone and uterus. Raloxifene is another SERM that inhibits bone resorption and prevents osteoporosis. The mechanism of this differential activity is not well understood. It is possible that different tissues express ER isoforms with different ligand affinities and different tissue levels of transcription factors that interact with the facilitated ER function.
While GR binds to its cis-acting element as a homodimer, other nuclear hormone receptors bind to their cis-acting elements as heterodimers with a common nuclear receptor monomer called RXR. For example, the retinoic acid receptor (RAR) forms the complex RAR-RXR in order to bind the retinoic acid response element. The receptors for vitamin D, thyroid hormone and peroxisome proliferators also form heterodimers with RXR. Since nuclear transport is not regulated for heterodimers as it is for GR, they are regulate by the binding of the RXR receptor. The endogenous ligand for RXR is 9-cis retinoic acid. In the prescence of ligand, the nuclear receptors heterodimers can direct hyperacetylation of histones, enhancing transcriptional expression. The N-terminus activation domain can also associate with additional transcriptional factors.
Peroxisome proliferator activated receptors (PPAR) respond to ligands that are fatty acid derivatives and can regulate expression and cell growth, differentiation and apoptosis. Activated PPARs bind to the PPAR response element (PPRE). PPAR ligands include natural molecules like linoleic acid, arachidonic acid and prostaglandin J2, and synthetic molecules like Wy14,643 and clofibrate. Most PPAR ligands have a carboxylic acid group and a large hydrophobic domain.
There are different PPAR isoforms: a, b and g. PPARa is expressed in a variety of tissues and stimulates oxidation of fatty acids by increasing the proliferation of peroxisomes and inducing enzymes in the mitochondria important for fatty acid metabolism. PPARg is important for differentiation of adipocytes. Thiazolidinedione (TZD) is an antidiabetic drug that decreases insulin resistance by antagonizing PPARg.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Glucocorticoid Receptors
 Cortisol
and other glucocorticoids affect fuel metabolism by differentially activating
liver, muscle and adipose cells. Glucocorticoids stimulates lipolysis in adipocytes
and release of amino acids from muscle protein. In the liver, cortisol stimulates
gluconeogenesis and glycogen synthesis. Glucocorticoids also decrease glucose
utilization in adipocytes and muscle cells. In this manner, cortisol mediates
long-term stress response by transcriptional control. In contrast, epinephrine
mediates release of glucose from the liver as a short-term response to stress.
Cortisol
and other glucocorticoids affect fuel metabolism by differentially activating
liver, muscle and adipose cells. Glucocorticoids stimulates lipolysis in adipocytes
and release of amino acids from muscle protein. In the liver, cortisol stimulates
gluconeogenesis and glycogen synthesis. Glucocorticoids also decrease glucose
utilization in adipocytes and muscle cells. In this manner, cortisol mediates
long-term stress response by transcriptional control. In contrast, epinephrine
mediates release of glucose from the liver as a short-term response to stress.
The glucocorticoid receptor (GR) is expressed in all tissue types. The inactive GR is normally bound to a large protein complex that includes hsp70 and hsp90. When glucocorticoids bind GR they facilitate the dissociation of hsp90. This exposes a DNA binding domain and a nuclear localization signal that allows translocation through the nuclear pore complex.

In the nucleus, GR dimerizes and binds glucocorticoid responsive element (GRE) sequences in the promoter of regulated genes. The trans-acting elements of GR contain a zinc finger DNA binding domain, flanked by transcriptional control domains that ensure GR binds to GRE and not to other cis-acting elements that may bind zinc fingers.
GR functions as either an activator or an inhibitor of transcription. The magnitude of the response depends on the number of GREs and their position relative to the transcriptional start site. Activation of transcription can involve interaction of the GRE tras-activation domain with basic transcription factors such a TFIIB. Such interactions can stabilize the preinitiation complex on the promoter and thus enhance transcription.
Alternatively, GR binding at GRE sequences can alter the chromatin structure at the promoter by inducing histone hyperacetylation, exposing previously masked sequences to bind by additional transcription factors. Hyperacetylation of histone N-terminals activate transcription. The Tau1 domain of GR interacts with a multisubunit histone acetyl transferase complex, inducing hyperacetylation of histone N-terminals that face the outer surface of the nucleosome. This local disruption of chromatin structure facilitates access of general transcription factors. Repression of transcription by nuclear receptors can involve deacetylation of histones and stabilization of chromatin.
<draw>
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
![]()
![]() Continue
to "Cell and Tissue Structure" or take
a test: [T1] [T2] [T3].
Continue
to "Cell and Tissue Structure" or take
a test: [T1] [T2] [T3].
Need more practice? Answer the following review questions:
Questions not yet available