Cellular and Molecular Biology Topics
![]()
![]()
The plasma membrane consists primarily of phospholipids and proteins. The phospholipids are arranged in two parallel rows, forming a bilayer. The phospholipids molecule consists of a polar, phosphate-containing “head” that mixes with water and a nonpolar fatty acid “tail” that does not mix with water. Molecules are oriented in the lipid bilayer so that heads face outward on either side and tails form a hydrophobic core.
Plasma membrane proteins are classified into two categories: integral and peripheral. Integral proteins are embedded in the lipid bilayer among the fatty acid tails. Some lie at or near the inner or outer membrane surfaces, while others penetrate the membrane completely. Some integral proteins form channels through which substances can be transported into and out of the cell. Peripheral proteins are loosely bound to he membrane surface.
 The
ability of the membrane to allow certain substances to enter and exit but restrict
passage of others is called selective permeability. The permeability of the
membrane is a function of several factors: size of the molecules, their lipid
solubility, charge on ions and the presence of carrier molecules. A pure artificial
phospholipids bilayer is permeable to small hydrophobic molecules and small
uncharged polar molecules, slightly permeable to water and urea, and impermeable
to ions and large uncharged polar molecules.
The
ability of the membrane to allow certain substances to enter and exit but restrict
passage of others is called selective permeability. The permeability of the
membrane is a function of several factors: size of the molecules, their lipid
solubility, charge on ions and the presence of carrier molecules. A pure artificial
phospholipids bilayer is permeable to small hydrophobic molecules and small
uncharged polar molecules, slightly permeable to water and urea, and impermeable
to ions and large uncharged polar molecules.
The processes involved in membrane transport may be classified as either passive or active. In passive processes, substances move across the cell membrane without an expenditure of energy because the substance moves on its own, down its concentration gradient, from an area of high concentration to an area of low concentration. In active processes, the cell uses energy in moving the substance across the membrane because the substance moves against its concentration gradient, from an area of low concentration to an area of high concentration.
Take Quiz: [Q1] [Q2] [Q3]
Back to Basics: Physiology
Passive transport processes include diffusion and facilitated diffusion. Diffusion occurs when the net movement of a substance is from a region of higher concentration to a region of lower concentration until the molecules are evenly distributed. At the point were they move in both directions at an equal rate, there is no net diffusion and the system is said to be in equilibrium. The difference between high and low concentrations is the concentration gradient.

The relative diffusion rate of a substance across the plasma membrane bilayer is proportional to its concentration gradient across the bilayer and to its hydrophobicity. The hydrophobicity of a substance is measured by its partition coefficient K:
K = Cm / Caq
where Cm is the concentration inside the hydrophobic core of the bilayer and Caq is the concentration in the aqueous solution. The partition coefficient is a measure of the relative affinity of a substance for lipid versus water: the higher the substance’s K, the more lipid soluble it is.
The rate of diffusion across the bilayer is given by a modification of Fick’s Law, which states that “the diffusion rate across the membrane (dn/dt) is directly proportional to the permeability coefficient (P), the difference in concentrations and the area (A):
dn/dt = PA(C1 – C2)
The permeability coefficient P accounts for the membrane thickness (x), diffusion coefficient (D) and the solubility of solute in membrane:
P = KD/x.
 The
tickness of the hydrophobic interior of all phospholipids bilayers is about
2.5 to 3 nm, and the diffusion coefficient is the same for all substances. Therefore
differences in the rate at which molecules diffuse across membranes depends
largely on differences in the partition coefficient.
The
tickness of the hydrophobic interior of all phospholipids bilayers is about
2.5 to 3 nm, and the diffusion coefficient is the same for all substances. Therefore
differences in the rate at which molecules diffuse across membranes depends
largely on differences in the partition coefficient.
Facilitated diffusion is a passive process that enables amino acids, nucleosides, sugars and other small molecules enter and leave cells down their concentration gradient via the assistance of uniporters. Uniporters are equally able to catalyze net movement in either direction, according to the concentration gradient. Each uniporter transport only a single species of molecule or a single group of closely related molecules.
Similar to enzymes, uniporters accelerate thermodynamically favored transport. The kinetics of uniporter-catalyzed transport reactions are characterized by a maximum transport rate (Vmax) and the substrate concentration at which half maximal transport occurs (Km). The lower the value of Km, the more tightly the substrate binds to the transporter and the greater the transport rate.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
 Membrane
proteins aid movement of materials across the plasma membrane. There are three
major classes: ATP pumps, channels and transporters.
Membrane
proteins aid movement of materials across the plasma membrane. There are three
major classes: ATP pumps, channels and transporters.
ATP pumps use energy from the hydrolysis of ATP to drive transport of a molecule against its concentration gradient. An example of an ATP pump is the Na+/K+ ATPase that keeps cells at a resting-state potential.
Channels allow the movement of ions or small molecules down their concentrationor electric potential gradients. They form a protein-lined passageway across the membrane through which multiple water molecules or ions move simultaneously, single file, at a very rapid rate. An example of a channel is the aquaporin protein. Phospholipid bilyers are somewhat permeable to water. Permeability to water is increased by water channel proteins named aquaporins (AQP). Unlike the water channels, which are constitutively open, many other channels are either ligand gated or voltage gated.
A transporter moves molecules either down their concentration gradient or against their concentration gradient, depending on the type of transporter. Examples of transporters are the several glucose transporters (GLUT).
 Three
types of transporters have been identified: uniporter, symporter and antiporter.
A uniporter transports one type of molecule down its concentration gradient.
A symporter couples the transport of a molecule down its concentration gradient
with the co-transport of another type of molecule against its concentration
gradient, both in the same direction. An antiporter couples the transport of
a molecule down its concentration gradient with the co-transport of another
type of molecule against its concentration gradient in the opposite direction.
Three
types of transporters have been identified: uniporter, symporter and antiporter.
A uniporter transports one type of molecule down its concentration gradient.
A symporter couples the transport of a molecule down its concentration gradient
with the co-transport of another type of molecule against its concentration
gradient, both in the same direction. An antiporter couples the transport of
a molecule down its concentration gradient with the co-transport of another
type of molecule against its concentration gradient in the opposite direction.
Symporters and antiporters move ions and small molecules against a concentration gradient using energy stored in the electrochemical gradient of either Na+ or H+. They do not directly use metabolilc energy (i.e. ATP) but depend on the gradient created by primary active transporters, like ATP pumps, therefore are called secondary active transporters.
 The
AE1 antiporter is the predominant integral protein of the mammalian erythrocyte
and plays a role in gas exchange. Waste CO2 released from
cells into the capillary blood diffuses across erythrocyte membranes. In its
gaseous form, CO2 dissolves poorly in the aqueous cytoplasm,
but the enzyme carbonic anhydrase converts it to the water-soluble bicarbonate
ion (HCO3-). The release of O2 from
hemoglobin induces a conformational change in the globin peptide that enables
a histidine side chain to bind the proton produced. The HCO3-
is then transported out of the erythrocyte in exchange for Cl- by AE1. The overall
direction of this reaction is reversed in the lungs.
The
AE1 antiporter is the predominant integral protein of the mammalian erythrocyte
and plays a role in gas exchange. Waste CO2 released from
cells into the capillary blood diffuses across erythrocyte membranes. In its
gaseous form, CO2 dissolves poorly in the aqueous cytoplasm,
but the enzyme carbonic anhydrase converts it to the water-soluble bicarbonate
ion (HCO3-). The release of O2 from
hemoglobin induces a conformational change in the globin peptide that enables
a histidine side chain to bind the proton produced. The HCO3-
is then transported out of the erythrocyte in exchange for Cl- by AE1. The overall
direction of this reaction is reversed in the lungs.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Osmolality and Water Transport
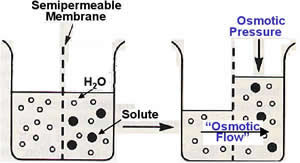 Water
tends to move across a membrane from a solution of low solute concentration
(high water concentration) to one of high solute concentration (low water concentration).
This process is known as osmotic flow. The hydrostatic pressure required to
stop the net osmotic flow across a membrane is defined as the osmotic pressure.
The osmotic pressure
of a solution depends on the number of impermeable particles it contains. The
concentration of impermeable particles can be calculated, and is called the
solution’s osmolality (mosm/L).
Water
tends to move across a membrane from a solution of low solute concentration
(high water concentration) to one of high solute concentration (low water concentration).
This process is known as osmotic flow. The hydrostatic pressure required to
stop the net osmotic flow across a membrane is defined as the osmotic pressure.
The osmotic pressure
of a solution depends on the number of impermeable particles it contains. The
concentration of impermeable particles can be calculated, and is called the
solution’s osmolality (mosm/L).
With regards to cell volume, the term tonicity is used to describe the osmolality of a solution relative to the cytoplasm. Solutions that have the same osmolality as the cytoplasm are isotonic, those with a greater osmolality are hypertonic and those with lesser osmolality are hypotonic. Animal cells will swell when placed in a hypotonic solution. Immersion of animal cells in hypertonic solutions will cause them to shrink.
Osmotic pressure is an important force in the movement of water between various compartments in the body. Simple rehydration therapy depends on osmotic gradient created by absorption of glucose and Na+. Treatment for cholera and other intestinal pathogens demands not only antibiotics but also rehydration with a solution of sugar and salt. The osmotic gradient set up by the absorption of glucose and sodium causes the bulk flow of water to enter the blood.
Phospholipid bilayers are somewhat permeable to water. Permeability to water is increased by water channel proteins named aquaporins (AQP). AQPs are tetramers of identical 28 kDa subunits, each containing six transmembrane alpha helices that form three pairs of homologs. The channel through which water moves is thought to be lined by eight alpha helices, two from each subunit.

AQPs are expressed in abundance in erythrocytes, kidney cells, and other that exhibit high water permeability. AQP2 is exclusively expressed in the principal cells of the collecting ducts and is predominantly regulated by vasopressin. Disregulation of aquaporins, and especially AQP2 will lead to water balance disorders. Reduced expression and/or lack of AQP2 are seen in conditions associated with extreme water loss like diabetes insipidus and renal failure. In contrast, increased expression and/or enhanced apical plasma membrane targeting of AQP2 are seen in conditions associated with water retention, like severe congestive heart failure and pregnancy.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
 There
are four principal classes of ATP-powered pumps: P-class, F-class, V-class and
ABC superfamily. The P-, F- and V-classes transport only ions. The ABC superfamily
transport small molecules as well as ions. All of these classes have one or
more ATP-binding sites and they only hydrolyze ATP if a substrate is simultaneously
being transported. Nerve and kidney cells expend up to 25% of their ATP produced
powering these pumps. Erythrocytes expend up to 50% of their ATP for this purpose.
There
are four principal classes of ATP-powered pumps: P-class, F-class, V-class and
ABC superfamily. The P-, F- and V-classes transport only ions. The ABC superfamily
transport small molecules as well as ions. All of these classes have one or
more ATP-binding sites and they only hydrolyze ATP if a substrate is simultaneously
being transported. Nerve and kidney cells expend up to 25% of their ATP produced
powering these pumps. Erythrocytes expend up to 50% of their ATP for this purpose.
The P-class pumps consist of a transmembrane catalytic alpha-subunit, which contains an ATP-binding site, and usually smaller beta-subunit which may have regulatory functions. Many are tetramers composed of two alpha and two beta subunits. During the transport process, at least one of the alpha subunits is phosphorylated and ions are thought to move through the phosphorylated subunit. This class includes the Na+/K+ ATPase, several Ca+ ATPases and the H+ ATPase.
 F-
and V-class pumps contain at least three kinds of transmembrane domains (a,
b and c) and five kinds of cytoplasmic domains (alpha, beta, gamma, delta and
epsilon). They transport only protons.
F-
and V-class pumps contain at least three kinds of transmembrane domains (a,
b and c) and five kinds of cytoplasmic domains (alpha, beta, gamma, delta and
epsilon). They transport only protons.
V-class pumps generally function to maintain the low pH of acidic vesicles by pumping protons from the cytoplasm into the vesicle. The plasma membrane of certain acid-secreting cells contains an almost crystalline array of V-class H+ ATPases.
F-class pumps are found in bacteria, mitochondria and chloroplasts, and function to power the synthesis of ATP from ADP and phosphate by movement of protons from the exoplasmic to the cytoplasmic face of the membrane.
 The
ABC superfamily consists of four “core” domains: two transmembrane domains (T)
forming the passageway and two cytoplasmic ATP-binding domains (A). This class
includes more than 100 different transport proteins specific for a single substrate
or a group of substrates including ions, sugars, peptides, polysaccharides and
even proteins.
The
ABC superfamily consists of four “core” domains: two transmembrane domains (T)
forming the passageway and two cytoplasmic ATP-binding domains (A). This class
includes more than 100 different transport proteins specific for a single substrate
or a group of substrates including ions, sugars, peptides, polysaccharides and
even proteins.
The multi-drug resistant transport (MDR) protein known as MDR1 is an ABC superfamily ATP pump that exports a large variety of small hydrophobic molecules, including drugs, from the cytosol to the extracellular fluid. Eaxh transmembrane domain contains six helices and two cytoplasmic ATP binding domains. MDR1 is expressed in abundance in liver, intestines and kidneys, sites from which natural toxins are removed from the body. It has the ability to transport drugs whose structure is similar to such toxins. Tumors derived from these cell types frequently are resistant to virtually all chemotherapeutic agents presumably because the tumor exhibits increased expression of these proteins.
The cystic fibrosis transmembrane regulator (CFTR) protein is a ABC superfamily ATP pump necessary for normal lung and digestive processes. Normally, it secretes Cl-, and Na+ follows to maintain electrical neutrality. The baddition of Na+ and Cl- to the mucus layer lining the airways pulls water into the mucus, necessary for normal pulmonary function. Cystic fibrosis patients have a defective CFTR, which results in a thick, sticky mucus lining that blocks small airways and hinders normal bacterial removal.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Two important P-class pumps are the Ca2+ ATPase and the Na+/K+ ATPase. The plasma membrane of most cells contain Ca2+ and Na+/K+ ATPases that transport Ca2+ and Na+ out of the cell and K+ into the cell, against their electrochemical gradients. ATP pumps help maintain the concentration of Na+ and free Ca2+ ions in the cytoplasm at very low levels.
Ca2+ ATPases contain the Ca2+ binding regulatory protein calmodulin, which when bound to Ca2+ allosterically activates the ATPase to export Ca2+ ions from the cell. Besides the plasma membrane Ca2+ ATPase, muscle cells contain a second, different ATPase that transports Ca2+ from the cytoplasm into the sarcoplasmic reticulum (SR). This transporter makes up more than 80% of the integral protein in SR membranes.
The steps of Ca2+ ATPase activation are as follows:

The Na+/K+ ATPase is a tetramer of subunit composition alpha2beta2. The beta subunit is required for newly synthesized alpha subunits correct folding, but apparently is not involved directly in ion pumping. The overall process of transport moves three Na+ ions out and two K+ ions into the cell per ATP. The mechanism is similar to the muscle Ca2+ ATPase, except that ions are pumped in both directions across the membrane:
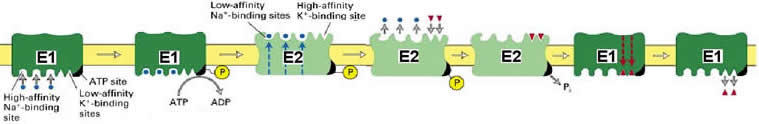
 In
cardiac muscle, an action potential stimulates the release of Ca2+ from the
SR via a voltage-gated channel. In addition, Ca2+ itself can induce Ca2+ release
through ligand-gated channels.
In
cardiac muscle, an action potential stimulates the release of Ca2+ from the
SR via a voltage-gated channel. In addition, Ca2+ itself can induce Ca2+ release
through ligand-gated channels.
The action potential induces depolarization of the cell surface, opening Ca2+ channels through which a strong inward current flows. The end result is action/myosin activation and contraction.
Cardiac muscle cells have a Ca2+ ATPase and a Na+/Ca2+ antiporter that normally maintain a low cytoplasmic Ca2+ concentration. The movement of three Na+ ions is required to power export of one Ca2+ against a 10,000 fold concentration gradient.
The drugs ouabain and digitalis increase the force of heart muscle contraction by inhibiting the Na+/K+ ATPase driving the Ca2+/Na+ antiporter, thus allowing decreasing the export of Ca2+ ions and increasing cytoplasmic concentration. With a higher intracellular baseline Ca2+ concentration, depolarization occurs more readily and contraction more strongly.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
In healthy cells, the specific ionic composition of the intracellular fluid differs greatly from that of the extracellular fluid. Na+ and Ca2+ ion concentrations are much higher outside the cell than inside. K+ concentration is much higher inside the cell than outside. Both passive and active transport are coordinated in living cells to generate and maintain these ionic gradients. As a consequence, healthy cells do not equilibrate with the but rather exist in a steady-state with the extracellular fluid.
The rate and extent of ion transport across membranes is influenced not only by the ion concentration on the two sides of the membrane but also the voltage of electrical potential that exist across the membrane. The electric potential depends on the charge gradient across the membrane. The sum of this two forces, concentration and charge gradients, is the electrochemical potential difference Du:
Du (x) = RT ln [x]i / [x]o + zF (Ei + Eo)
where R is the gas constant (joules/mol·K), F is Faraday’s constant (coloumbs/mol), T is the absolute temperature and z is the charge of the ion x.
The first term, RT ln [x]i / [x]o, is the tendency for ion x to move from the inside to the outside of the cell because of the concentration difference. The second term, zF (Ei – Eo), is the tendency for the ion to move from the inside to the outside of the cell because of the electrical potential difference.
Ions will tend to move from higher to lower electrochemical potential. If Du is positive, ions will move from the inside to the outside of the cell. If Du is negative, ions move from the outside to the inside. If Du is zero, there is no net movements of ions either way, and the particular ion is said to be in electrochemical equilibrium.
At equilibrium Du = 0, therefore:
0 = RT ln [x]i / [x]o + zF (Ei + Eo)
The Nerst equation is obtained by solving for the difference in electrical potential (Ei – Eo):
Ei + Eo = -RT/zF - ln [x]i / [x]o
(Ei + Eo) is commonly written as Eion, for example EK is the potassium potential. The units of RT/F are joules/coloumb or volts. In electrophysiology, millivolts are more often used. The solution of the Nerst equation is easier when base 10 logaritms are used intead of natural logarithms:
Ex = -2.203 RT/zF · log [x]i / [x]o = -61/z · log [x]i / [x]o
The Nerst equation predicts the membrane potential that would be set up by a specific ion at equilibrium. Thus the equation can be used to prove whether specific ions are at equilibrium.
The resting membrane potential is the electrical potential difference across the cell membrane of a normal living cell in its unstimulated state. It can be measured directly with a microelectrode inserted into the cell and a reference electrode in the extracellular fluid, and is determined by those ions that can cross the membrane and are prevented from attaining equilibrium by active transport processes.
The Goldman equation gives the value of the membrane potential when all permeable ions are accounted for:
Em = RT
· PK[K+]o + PNa[Na+]o
+ PCl[Cl-]i
F
PK[K+]i + PNa[Na+]i
+ PCl[Cl-]o
where P represents the permeability of the membrane to the specified ion. Plasma membranes of most cells are much more permeable to potassium ions than to any other ion. When solving the Goldman equation, the result is very similar to that of the Nerst equation fro potassium. This illustrates how in most cells the resting potential is dominated by K+ because the cell membrane is more permeable to this ion compared to others.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Facilitative glucose transporting proteins (GULT) are uniporters arranged in 12 transmembrane segments to form a pore. Both the N-terminus and C-terminus face the intracellular compartment. A large extracellular loop near the N-terminus is glycosylated in the mature protein. Currently 12 GLUT isoforms are known.
The GLUT protein alternates between two conformational states: with the glucose-binding site facing the outside of the membrane or facing the cytosol.

Although the GLUT1 protein is ubiquitously expressed, the other glucose uniporters, GLUT2 to GLUT 12, have specific tissue distributions. One of the major biological responses following insulin stimulation is a marked increase in the rate of glucose transport in muscle and adipose tissue. This results from an insulin-dependent translocation of a muscle/adipose tissue specific GLUT4 from an intracellular storage vesicles to the plasma membrane. The failure of GLUT4 to respond to normal levels of insulin is associated with diabetes, obesity, aging, ad a sedentary lifestyle.
Most internal and external body surfaces of animals are covered with a layer of epithelial cells (epithelium). These cells are polarized because one side differs in structure and function from the other. The portion of the membrane facing the environment is the apical surface. The rest of the plasma membrane is the basolateral surface and mediates transport of substances from the cell to the surrounding extracellular fluid. The basolateral surface forms junctions with adajcent cells and the basal lamina through integral membrane proteins.
In the intestines, the apical surface is specialized for absorption and composed of microvillus, finger-like projections (~100 nm in diameter) collectively known as the brush border. The brush border greately increases the area of absorptive capacity. It contains a loose network of oligosacharide side chains of integral membrane proteins, glycolipids and enzymes known as glycocalyx.
 Movement
of glucose and amino acids from the intestinal lumen into the blood is a two-stage
process:generation of gradients at the basolateral membrane and co-transport
of glucose at the apical membrane.
Movement
of glucose and amino acids from the intestinal lumen into the blood is a two-stage
process:generation of gradients at the basolateral membrane and co-transport
of glucose at the apical membrane.
Activity of the Na+/K+ ATPase in the basolateral membrane generates Na+ and K+ concentration gradients. The K+ gradient generates an inside negative membrane potential.
Both the Na+ concentration gradient and the membrane potential are used to drive the uptake of glucose from the intestinal lumen by the 2Na+/1glucose symporter located in the apical membrane (SGLT1).
Glucose leaves the cell via facilitated diffusion catalyzed by GLUT2, a glucose transporter localized in the basolateral membrane.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
Several mechanism use vesicles to transport macromolecules or larget particles across membranes: Phagocytosis, endocytosis and exocytosis.

Phagocytosis mediates ingestion of large particles or microorganisms by cells. This usually occurs in specialized cells by engulfing of particle by the plasma membrane.
 Endocytosis
involves invagination of the membrane, collecting molecules from the environment.
This may occur in two ways: pinocitosis or receptor-mediated endocytosis. Pinocytosis
involves fluid phase non-specific utake of extracellular material. Receptor
mediated uptake of specific molecules ocurs by forming a coated pit.
Endocytosis
involves invagination of the membrane, collecting molecules from the environment.
This may occur in two ways: pinocitosis or receptor-mediated endocytosis. Pinocytosis
involves fluid phase non-specific utake of extracellular material. Receptor
mediated uptake of specific molecules ocurs by forming a coated pit.
Budding of coated vesicles is initiated by recruitment of a small GTP-binding protein to a patch on the membrane. The complexes of coat and adaptor proteins bind to the cytoplasmic domains of membrane cargo and receptor proteins.
Exocytosis is responsible for cellular export and can be either constitutive or regulated.
Take Quiz: [Q1] [Q2] [Q3]
Advance Topics:
![]()
![]() Continue
to "Cell to Cell Communication" or
take a test: [T1] [T2] [T3].
Continue
to "Cell to Cell Communication" or
take a test: [T1] [T2] [T3].
Need more practice? Answer the following review questions:
Questions not yet available