
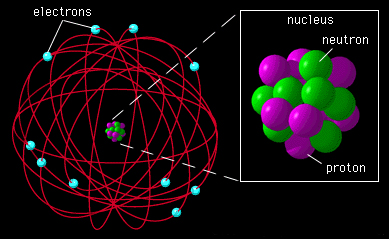
Also called the "nuclear atom" the Rutherford atom displaced the Thomson atom and was an essential step that led to the quantum theory.
The detailed experiments by Geiger and Marsden in 1913 showed beyond a shadow of doubt that the picture of a small, massive nucleus at the center of a much larger electronic structure was correct. Several points carried over from the Thomson atom, such as the existence of electrons in the nucleus, also discovered later.
The problem of the "Rutherford" atom was that all electron orbits were possible and it was constantly radiating all wavelengths of electromagnetic waves. This problem was solved with the discovery of quantum mechanics by Max Planck, Albert Einstein, Niels Bohr and others in the early 20th century.