Quantum Chemistry for the Beginners
Lesson II :: Quantum Chemistry of Atoms
Lesson I :: Basic Concepts of Quantum Chemistry
2.1. The simplest case of the hydrogen atom and the hydrogen-like atomic systems:
The hydrogen atom, with one proton acting as the atomic
nucleus and only one electron, is obviously akin to some other particular
atomic ions (He+ ion, Li2+ ion, Be3+ ion etc.),
as each of the above contains only one nucleus (that within it containing Z
number of protons, where Z -- e.g., 1, 2, 3, 4 -- is the atomic
number) and only one electron. So the H-atom and the aforesaid
one-electronic atomic ions are together called 'hydrogen-like atomic systems'.
Having only one nuclei and only one electron, such simple systems provide exact,
direct solutions of the Schrodinger equation. To tell you the complete truth, for any such systems the complete Schrodinger equation is actually
separable into two simpler Schrodinger equations -- one for the translational
motion (i.e., for movement from one location to another) of the
atom/ion/molecule as a whole, and the other one for the motion of the
electron and the nucleus relative to each other (this, however, can be simply
visualised as just the motion of the electron relative to the nucleus -- the
electron being much lighter than the nucleus). The latter Schrodinger equation,
for the electronic motion, can be simply stated as ĤY = iħ(∂Y/∂t),
with the corresponding time-independent Schrodinger equation being stated as Ĥy = Ey
(E being the electronic energy of the atom), where the Hamiltonian operator Ĥ
for a hydrogen-like system is simply {ħ2/(2me)}.{(∂2/∂xe2)
+ (∂2/∂ye2) + (∂2/∂ze2)}
Ze2/(4peore). It
should be noted that here y = y(q)
i.e., y(xe,ye,ze) or y(re,qe,fe)
in
the last equation is the electronic wavefunction, and that an electronic
wavefunction for a one-electron atomic system is also called an orbital (say,
the 1s orbital). [However, for a multi-electron atom, it so happens that the electronic
wavefunction is a sort of a product of several (occupied) electronic orbitals --
that idea is detailed
later.]
Note: Have you noted the term 'atomic ion'? An atomic ion
(whether cation or anion, e.g., Na+ or Cl) can be
formed from one atom by loss or addition of only electron(s), and is thus
distinct from molecular ions (e.g., H2+, N2)
and multi-atom radical ions (e.g., NH4+, SO42 ).
For sake of truth, it may now be told to you (should it really be?) that
the above use of me (mass of electron) in the factor {ħ2/(2me)}
multiplying the Laplacian is only an approximation, which quantity should,
exactly speaking, be replaced with the 'reduced mass' m
equalling me.mnuc/(me+mnuc) -- but
this simplification introduces rather too minor an error (as obviously m
≈ me -- can you recognise how?). More importantly, note
the appearance of Z in the potential-energy expression
Ze2/(4peore),
which was absent (there Z was unity!) in the earlier expression in Lesson I
given about only the H-atom. Here, however, we give a general expression true
for H-atom, He+ ion, Li2+ ion etc. i.e., for all one-electron
one-nucleus systems, and so here Z does appear [here (e) is the charge of the
electron, (+Ze) is the charge of the nucleus, their distance is re,
and so the potential-energy expression, as per Coulomb's law, is
e.Ze/(4peore)]. Further, we
choose the electron to be at the point (x,y,z) -- obviously equivalent to (r,q,f)
-- while the nucleus is chosen to be at the origin O of the coordinate system.
This means that (∂2/∂xe2)
+ (∂2/∂ye2) + (∂2/∂ze2)
can be simplified as being (∂2/∂x2)
+ (∂2/∂y2) + (∂2/∂z2)
while Ze2/(4peore)
can be simplified as
Ze2/(4peor). Now expressing
the Laplacian ![]() i.e., (∂2/∂x2
+ ∂2/∂y2 + ∂2/∂z2)
as ∂2/∂r2
+ (2/r)∂/∂r
+ (1/r2)∂2/∂q2 +
(1/r2)cotq.∂/∂q +
(1/r2)cosec2q.∂2/∂f2,
we get the electronic Schrodinger equation fully spelt out in terms of (r,q,f)
coordinates only, and can now easily proceed to solve it.
i.e., (∂2/∂x2
+ ∂2/∂y2 + ∂2/∂z2)
as ∂2/∂r2
+ (2/r)∂/∂r
+ (1/r2)∂2/∂q2 +
(1/r2)cotq.∂/∂q +
(1/r2)cosec2q.∂2/∂f2,
we get the electronic Schrodinger equation fully spelt out in terms of (r,q,f)
coordinates only, and can now easily proceed to solve it.
2.2. How did we arrive at the familiar hydrogen-atom electronic
wavefunctions?
The aforesaid (time-independent) electronic Schrodinger equation (expressed in (r,q,f) coordinates) can be further separated into two daughter differential-equations -- a radial equation (involving only r coordinate) and an angular equation (involving only q and f coordinates -- I don't want to tell what exactly are these two equations). The radial equation when solved (via a complicated procedure which you better be ignorant of) gives a function of r as its solution -- this function is called the radial wavefunction, denoted as Rnl(r). The angular equation when solved (via a comparably complicated procedure) gives a function of q and f as its solution -- this function is called the angular wavefunction or the 'spherical harmonics', denoted as Ylm(q,f). The complete electronic wavefunction for the hydrogen-like atomic system, Ynlm(r,q,f) is the product of the radial and the angular wavefunctions, i.e., Ynlm(r,q,f) = Rnl(r).Ylm(q,f) [the wavefunction Ynlm is the eigenfunction of the Hamiltonian operator in the aforesaid electronic Schrodinger (time-independent) equation with the energy eigenvalue E = (13.6Z2/n2) eV]. You are probably guessing by now (yes, this is indeed so) that the subscripts n, l and m means, respectively, the principal, the azimuthal and the magnetic quantum numbers of the atomic orbital (occupied by the lone electron in the hydrogen-like atomic system). From the symbolism used, it is clear to you that the radial wavefunction depends only on the principal and the azimuthal quantum numbers, whereas the angular wavefunction depends only on the azimuthal and the magnetic quantum numbers. Also, there is nothing mysterious about these three quantum numbers appearing in atomic electronic wavefunctions with definite rules (e.g., l < n) binding them -- these quantum numbers, along with the well-known limitations binding their values, arise simply while solving the radial and the angular equations (in a process similar to the appearance of integral quantum-number - n - values for the aforesaid particle in a one-dimensional box system).
The radial and the angular wavefunction must remain
separately normalised, so that the complete wavefunction Ynlm(r,q,f)
remain normalised. This means that the normalisation requirement 0∫+∞0∫2p0∫p |Ynlm(r,q,f)|2 r2
sinq dq df
dr = 1 i.e., {0∫+∞|Rnl(r)|2.r2
dr}.{ 0∫2p0∫p
|Ylm(q,f)|2
sinq dq df}
= 1, should imply two specific sub-requirements (i) 0∫+∞|Rnl(r)|2. r2
dr = 1 and (ii) 0∫2p0∫p
|Ylm(q,f)|2
sinq dq df
= 1. It is these two requirements that decide what'll be the exact forms of
these two wavefunctions. As an example, the 2po hydrogen-like wavefunction (i.e.,
Y210) is {Z5/(32pao5)}1/2 r exp{Zr/(2ao)}.cosq,
and for this complete wavefunction the radial and the angular wavefunctions
(i.e., R21 and Y10) are, respectively, {Z5/(24ao5)}1/2
r exp{Zr/(2ao)} and {3/(4p)}1/2 cosq.
[In other words, they can't be something like {Z5/(32pao5)}1/2 r exp{Zr/(2ao)}
and cosq respectively -- check for yourself whether
both the normalisation requirements are being separately satisfied by the former
(i.e., the valid) pair of radial and the angular wavefunctions.]
Problem: Find the angular and the radial wavefunction corresponding to the
hydrogen-like 1s-state wavefunction
Y100. Ans: We knew (Section 1.5) that Y100
= ({Z3/(pao3)}1/2
exp(Zr/ao), and now we know that Y100(r,q,f)
= R10(r).Y00(q,f).
However, this function Y100 has no
dependence on q and f, and
so its (q,f) dependent
part -- i.e., Y00(q,f)
-- must be nothing but a constant. Let it be NY, a real positive
constant -- just like a normalisation constant. This means that the aforesaid
normalisation requirement for Ylm(q,f)
function simply implies 0∫2p0∫p
NY2
sinq dq df
= 1, i.e., NY2
= 1/0∫2p0∫p
sinq dq df.
From earlier experience (Section 1.5), we know the integral at the denominator to be 2 x 2p
= 4p, implying that NY = (1/4p)1/2
i.e., Y00(q,f)
= (1/4p)1/2. Now, utilising the relation Y100(r,q,f)
= R10(r).Y00(q,f),
we may immediately get R10(r) = Y100(r,q,f)/
Y00(q,f) = 2(Z3/ao3)1/2
exp(Zr/ao) -- isn't it?
2.3. The forms of the common radial and angular
wavefunctions:
Let us now put forward the mathematical expressions (check the already known ones) of some of the radial and angular wavefunctions (i.e., Rnl and Ylm) as well as the complete wavefunctions Ynlm(r,q,f) associated with all the hydrogen-like atomic orbitals corresponding to the 1s, 2s, 2p, 3s, 3p and 3d subshells. We'll then take note of some of their interesting characteristics.
Table 2.1: The Radial Wavefunctions Rnl(r)
| Radial Wavefunction Name | Associated Subshell |
Functional Form of Rnl(r) |
| R10(r) | 1s | 2(Z3/ao3)1/2 exp(Zr/ao) |
| R20(r) | 2s | {Z3/(2ao3)}1/2 {1Zr/(2ao)} exp{Zr/(2ao)} |
| R21(r) | 2p | (1/2){Z5/(6ao5)}1/2 r exp{Zr/(2ao)} |
| R30(r) | 3s | (2/3){Z3/(3ao3)}1/2 {12Zr/(3ao)+2Z2 r2/(27ao2)} exp{Zr/(3ao)} |
| R31(r) | 3p | (8/27){Z3/(6ao3)}1/2{Zr/ao Z2r2/(6ao2)} exp{Zr/(3ao)} |
| R32(r) | 3d | (4/81){Z7/(30ao7)}1/2 r2 exp{Zr/(3ao)} |
Table 2.2: The Angular Wavefunctions Ylm(q,f)
| Angular Wavefunction Name | Associated Orbital Type |
Functional Form of Ylm(q,f) |
| Y00(q,f) | s | {1/(4p)}1/2 |
| Y10(q,f) | po | {3/(4p)}1/2 cosq |
| Y11(q,f) | p+1 | {3/(8p)}1/2 sinq exp(if) |
| Y1,1(q,f) | p1 | {3/(8p)}1/2 sinq exp(if) |
| Y20(q,f) | do | {5/(16p)}1/2 (3cos2 q 1) |
*Note: exp(if) = cosf
+ i sinf, exp(if)
= cosf i sinf
where i = (1)1/2. Also note that
Ylm(q,f) is
always of the form Ylm(q,f)
= g(q).exp(±imf), where
g(q) is a function of q
only.
Table 2.3: The Complete Wavefunctions Ynlm(r,q,f)
= Rnl(r).Ylm(q,f)
| Complete Wavefunction Name | Corresp. Orbital |
Functional Form of Ynlm(r,q,f) |
| Y100(r,q,f) | 1s | {Z3/(pao3)}1/2 exp(Zr/ao) |
| Y200(r,q,f) | 2s | {Z3/(8pao3)}1/2 {1Zr/(2ao)} exp{Zr/(2ao)} |
| Y210(r,q,f) | 2po | (1/4){Z5/(2pao5)}1/2 r exp{Zr/(2ao)}cosq |
| Y211(r,q,f) | 2p+1 | (1/8).{Z5/(pao5)}1/2 r exp{Zr/(2ao)} sinq exp(if) |
| Y2,1,1(r,q,f) | 2p1 | (1/8).{Z5/(pao5)}1/2 r exp{Zr/(2ao)} sinq exp(if) |
| Y300(r,q,f) | 3s | (1/3){Z3/(3pao3)}1/2 {12Zr/(3ao)+2Z2 r2/(27ao2)} exp{Zr/(3ao)} |
For wavefunctions or orbitals with an non-zero value of the magnetic quantum
number m, such as 2p+1 and 2p1 displayed above, we find
that the wavefunction is not real, i.e., complex (e.g., 2p+1 above
contains a complex factor cosf + i.sinf). Also, for
these orbitals the directional characteristics are not much specific, e.g., the 2p+1
function is not cylindrically symmetric around the x axis or the y-axis,
unlike the 2po function (also known as 2pz) which is
cylindrically symmetric around the z-axis (you probably intuitively follow what
the last clause means). So, for cases of non-zero values of m, we can form
new pairs of real-valued orbitals (What?
Are we God? But yes, we are allowed to do this particular limited operation!) with specific directional characteristics by
'linearly combining' the two orbitals with same values of n and l
but additive
inverse values of m. Thus from the 2p+1 and 2p1 orbitals,
we create the pair of linear combinations (1/2)1/2.(2p+1 +
2p1) and (1/2)1/2.(2p+1 2p1)/i
(here (1/2)1/2 arises as a normalisation constant). We find that the
first one is cylindrically symmetric around the x-axis [it has a functional form
N.x. exp{Zr/(2ao)}], whereas the second is cylindrically symmetric around the y-axis
[having
a functional form N.y. exp{Zr/(2ao)}]; so we call the first combination as 2px and
the second one as 2py. Similarly from the 3d+1 and 3d1
orbitals, we would get the combinations (3d+1 + 3d1)/21/2
and (3d+1 3d1)/21/2, while from 3d+2 and
3d2, we get the combinations (1/2)1/2.(3d+2 + 3d2)
and (1/2)1/2.(3d+2 3d2)/i. Judging
their directional characteristics, we choose to call the four new d-orbitals as
3dxz, 3dyz, 3dx2y2
& 3dxy (while the unchanged 3do orbital is called also
as 3dz2). Such real-valued and specific-direction orbitals are more
convenient for chemical discussions and understanding. Below we tabulate the three real wavefunctions belonging
to the 2p subshell.
Note: By calling the 2po function as cylindrically
symmetric around z-axis, we mean that when one moves away some fixed distance
perpendicularly from the axis, from a particular point on the axis, one comes
across the same value of the function.
Table 2.4: Some of the Real Wavefunctions Ynlm(r,q,f)
|
Real Orbital (Wavefunction) Name |
Corresponding Wavefunction Name |
Functional Form of the Wavefunction |
| 1s | Y1,0,0(r,q,f) | {Z3/(pao3)}1/2 exp(Zr/ao) |
| 2s | Y2,0,0(r,q,f) | {Z3/(8pao3)}1/2 {1Zr/(2ao)} exp{Zr/(2ao)} |
| 2pz | Y2,1,0(r,q,f) | (1/4).{Z5/(2pao5)}1/2 r exp{Zr/(2ao)}cosq |
| 2px | Y2,1,±1(r,q,f) | (1/4).{Z5/(2pao5)}1/2 r exp{Zr/(2ao)} sinq cosf |
| 2py | Y2,1,±1(r,q,f) | (1/4).{Z5/(2pao5)}1/2 r exp{Zr/(2ao)} sinq sinf |
2.4. Important characteristics of the radial and the angular wavefunctions:
The radial wavefunction Rnl(r) describes the variation of the complete wavefunction ynlm with the radial distance r. Now, so, let's see how Rnl varies with r. For all the s-subshells (i.e., for the l = 0 ones), Rnl starts with a high positive value at r = 0 (this is rather shocking to our common sense, indicating that for the s-subshells the probability of the electron to be at or very near the nucleus is high!) and gradually decreases as r increases up to a limit. For the 1s subshell, Rnl (= R10) always remains positive, and continually decreases with increasing r, asymptotically approaching zero as r tends to infinity (Fig. 2.1). However for 2s, Rnl (= R20) becomes negative as r becomes greater than 2ao/Z (Why? Find out from Table 2.1, also checking that R20 equals 0 at r = 2ao/Z), attaining a minimum for some value of r, and then increasing in algebraic value (but decreasing in magnitude) as r increasing further, finally asymptotically approaching zero as r tends to infinity (Fig. 2.1). Drawing a not-to-scale, exam-purpose plot of 3s is now easy for you (do try it!) -- R30 would first decrease from a high positive value, reaching zero and decreasing further till it reaches a negative minimum, then increasing, crossing zero on the way again and then reaching a positive maximum, then decreasing continually, finally asymptotically approaching zero (Fig. 2.1). Rnl for the non-s subshells, however starts with a zero value at r = 0 (more pleasing to our common sense), with Rnl increasing as r increases up to a limit, then decreasing from the maximum attained. For 2p and 3d subshells (i.e., for n l = 1), the said decrease with r is continual throughout, with asymptotic decrease of Rnl towards 0 (Fig. 2.1). However for 3p, 4p, 4d etc. subshells (with n l > 1), the decrease of Rnl leads to a negative-value minimum followed by increase with further increase of r (Fig. 2.1). [Figures reproduced from Sen*]

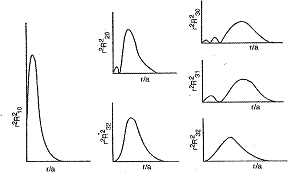
Fig.
2.1
Fig. 2.2
At the end of this discussion, let us now judge the number of intermediate (i.e., in between r = 0 and r = a) locations having zero value for the radial wavefunction Rnl -- such intermediate locations are called the radial nodes. The number of radial nodes for a radial wavefunction is n l 1, isn't it? (Do check it yourself from the above plots).
As the angular wavefunction Ylm(q,f) depends on two variables (i.e., q and f), that too angle-variables, similar two-dimensional (i.e., on-paper) plotting of Ylm versus its variables is unwarranted. Instead we try to visualise their functional variations. Y00 is a constant with no angular variations. Y10 is evidently maximum at q = 0 and minimum at q = p (but magnitude-wise maximum there also), but zero on the plane (i.e., xy plane) with q = p/2 (thus, this plane is a nodal surface for Y10). The effect of the radial nodes and the angular nodal surfaces result in nodal surfaces for the complete wavefunction Ynlm. Thus for Y200 (i.e., 2s orbital), there is one spherical nodal surface with radius 2ao/Z from the nucleus, whereas for Y200 (i.e., 2po orbital), there is one planar nodal surface which is the xy (i.e., XOY) plane. It can be shown (cf. Levine) that for each of the real hydrogenlike orbitals, the total number of nodal surfaces is n 1 (thus, 1s orbital has no nodal surface).
Let us now consider the probability of the electron being observed with the radial distance r lying between some given value r and (r+dr), irrespective of the direction from the nucleus (and so, irrespective of values of the angles q and f). We knew that the probability of the electron lying with r between r & (r+dr), q between q & (q+dq), and f between f & (f+df) is |y(r,q,f)|2 r2 sinq dq df dr = |R(r)|2 |Y(q,f)|2 r2 sinq dq df dr. So the probability that the electron lies with r between r & (r+dr), irrespective of the values of the angles q and f (or in other words within an annular region between two spheres with radius r and r+dr) is dpr = 0∫2p0∫p |R(r)|2 |Y(q,f)|2 r2 sinq dq df dr = |R(r)|2 r2 dr. 0∫2p0∫p |Y(q,f)|2 sinq dq df. As, we know, 0∫2p0∫p |Y(q,f)|2 sinq dq df always equals 1, so this probability dpr = |R(r)|2 r2 dr. However, as the radial wavefunction R(r) is always real (Table 2.1), |R(r)|2 further equals R2(r) or R2 (also denoted as Rnl2). Thus the probability dpr = r2 R2 dr = f(r) dr, where f(r) = r2 R2 (rhyme-like to remember, isn't it?) is called the radial distribution function (this name implying that the probability distribution along the radial distance r is given by this function). In contrast to the radial wavefunction Rnl(r), the radial distribution function r2 Rnl2(r) has zero value at r = 0 (obviously due to the r2 factor in it) even for the s-type wavefunctions, have higher values for larger values of r (due to the r2 factor), and is everywhere positive-valued (as Rnl(r) function remains squared here) -- check the r2 Rnl2(r) vs. r plots in Fig. 2.2.
2.5. Orbital energies in hydrogen atom and in the many-electron atoms:
In a hydrogen atom or in any other hydrogen-like atomic system
with only one electron (e.g., O7+ ion), the electron's energy (here
same as the orbital energy -- of the orbital occupied by the lone electron) is found to be Z2e2/(8peon2ao)
= (13.6Z2/n2) eV, also expressed as (Z2/2n2)EH
-- EH being the atomic unit of energy also called the Hartree,
equalling 27.2 eV. [Putting values of e = 1.602 x
1019 C, 1/4peo = 8.988 x 109
N m2 C2 and ao = 0.529 x 1010
m in the first expression, verify that it indeed equals (2.18Z2/n2)
x 1018 J = (13.6 Z2/n2) eV = (Z2/2n2)EH.
Let you also hear here that ao, the atomic unit of length, actually equals (4peo)h2/(4p2me2),
thus approximately equalling (4peo)h2/(4p2mee2)
-- may verify its given value (i.e., 0.529 Å) also.]
Problem: For Li2+ ion, what is the wavenumber 1/l
of the second line in the Balmer series? Ans: For hydrogenlike systems, the
wavenumber expression is, obviously, 1/l = Z2.Ry.(1/nl2
1/nu2). So here, as Z = 3 (Li2+ ion), nl
= 2 (Lyman series), nu = nl + 2 = 4 (the second line), we
get 1/l = 1.851 x 107 m1.
However, for a many-electron atom, its orbital energies
aren't given by the above expression (Z2/2n2)EH.
This is because an electron in any given orbital are greatly screened (shielded)
from the attraction of the nucleus by the presence of the other electrons, so
that the electron considered do not experience the full nuclear charge Z but
rather an effective nuclear charge Zef, less than Z by an screening
constant s (value of s
depends on the atom and on the orbital), i.e., Zef = Zs.
Thus the orbital energy actually becomes (Zef2/2n2)EH
= {(Zs)2/(2n2)}EH.
Screening or shielding arises because an electron in a
many-electron atom is simultaneously acted upon by the nucleus and the other
electrons. The nucleus, with its charge +Ze, attracts the electron to itself
whereas every other electron, with a charge e each, repels it. As the
electrons in an atom, in an averaged-out consideration, remain spherically
symmetrical around the nucleus, the net result of the nuclear attraction and the
repulsion by the other electrons is that the considered electron is pulled
towards the nucleus with a much weaker force compared to the actual nuclear attraction. This is screening (or the
screening effect), as a result of which the electron experiences an effective
nuclear charge Zef = Zs instead of the
actual nuclear charge Z.
Problem: What are the orbital energies of the 1s, the 2s and the
2p orbitals in an O atom, with the screening constant equalling (as per the well-known
Slater's rules) 0.30, 3.45 and 3.45 for the 1s, 2s and 2p orbitals
respectively. Ans.: Here Z = 8. For the 1s orbital, n = 1 whereas for the 2s and
2p orbitals n = 2. So, orbital energy for 1s is (7.72/2)EH
whereas for 2s and 2p it is (4.552/8)EH (actually the
2s & 2p energies should have been different, but Slater's rules is a rather
coarse approximation). Have you noted (verify the values)
that the orbital energies are invariably lower for larger-Z atoms e.g., that 2s
of O-atom would lie lower than 2s of H-atom? Note also that screening raises the
outer-orbital energies more (with larger s values)
than the inner-orbital ones.
There's another effect that causes, via the
screening effect, unequal variations in orbital energies. The s orbitals
penetrate, i.e., have appreciable function values, up to the region near the
nucleus, whereas the p and the d orbitals do not similarly penetrate up to the
near-nuclear region. Such varying penetration of different orbitals to the inner
region of the atom is called the penetration effect. Because of
penetration effect, the orbitals with differing values of the azimuthal quantum
number l are screened to different extent, thus producing unequal variations in
their energies. In particular, the orbital energies of the different subshells
of the same atomic shell (e.g., the 3s, 3p and 3d subshell of the 3rd shell --
i.e., the M shell) becomes different due to the combined action of the
screening and the penetration effects. As per the hydrogenlike-system
orbital-energy expression (Z2/2n2)EH, such
subshell energies such as the 3s, 3p & 3d energies would have been identical
(as Z and n values are same for all of them) -- they are indeed same in a H-atom
or in an one-electron atomic ion. However, as per the screening-corrected energy
expression {(Zs)2/(2n2)}EH,
these subshell energies in a many-electron atom are different, as the screening
constant s is different for the different subshells.
This difference in screening constant occurs because a lower-l subshell (e.g.,
3s) penetrates more into the inner core and thus are screened less (i.e., s
is smaller), whereas a higher-l subshell (e.g., 3d) penetrates less into the
inner core, with its electrons remaining more in the outer region, and thus are
screened more (i.e., s is larger). So for the 3s
subshell, the orbital energy (= {(Zs)2/18}EH)
is lower* whereas for the 3d subshell the orbital energy (= {(Zs)2/18}EH)
is higher (what's this 18?), and for 3p subshell it is in between (thus giving
the familiar energy variation 3s < 3p < 3d). For the d-subshells (and
obviously also for the f-ones), the subshell energy thus becomes so high that it
even becomes more than that of the s-subshell of the next shell -- thus energy
of the 3d subshell is greater than that of the 4s, and similar is the case with
the 4d and the 5s subshells.
* Note: You may chant, about the 3s subshell, the following
rhyme: Smaller s, bigger (Zs),
bigger (Zs)2, lower {(Zs)2/18}EH.
For the 3d one, it would be: Bigger s, smaller (Zs),
smaller (Zs)2, higher {(Zs)2/18}EH.
Problem: Invoke the Slater's rules to verify that for Ca atom, the 3d orbital
energy is indeed more than that of 4s (so that for Ca atom the ground electronic
configuration is [Ne]3s23p64s2 but not [Ne]3s23p63d2).
2.6. The two ways of representing shapes of electronic orbitals:
Atomic orbitals, and also molecular ones, are made of electronic wavefunctions or of probabilities, and so resemble rather coils of chimney smoke instead of solid dumbbells. Nevertheless, one can discuss their spatial shapes just as one discusses about the shapes of such hazy objects in daily life. There are two popular ways of representing the three-dimensional shapes of atomic orbitals, called the electron-dot representation and the boundary-surface representation.
In the electron-dot representation, the relative probability of observing the electron in a given location is represented by the density of dots in that location. Wherever the electron probability is more, the dots are more dense, and wherever that probability is less, the dots are sparse, as shown in the electron dot representation for the 1s, 2s and 2po orbitals (Fig. 2.3).
In the boundary-surface representation of the hydrogenlike orbital wavefunction y(r,q,f), closed surfaces (called boundary surfaces) are drawn that enclose atomic regions with high magnitude of y, and in such a way so that a fixed major part (say, 90%) of the total electron probability gets enclosed within such surfaces. To realistically represent the orbital shapes, they are drawn in a characteristic method, namely by plotting the magnitude |y(r,q,f)| along the radial distance r, assuming a fixed value of the variable r in the function y(r,q,f), versus differing values of the coordinate angles q and f indicating all possible directions. [Note: This is similar to plotting the value of a function f(x,y) along y axis versus differing values of x, assuming a fixed value for the variable y.] This procedure gives the familiar spherical shapes for representations of 1s and 2s orbitals as well as the familiar 'two touching spheres' representations for the 2po (or 2pz), 2px & 2py orbitals (Fig. 2.4). However, the boundary-surface representation of the orbital probability density |y|2 is more popular: in this representation, the square of the magnitude i.e., |y(r,q,f)|2 is instead plotted along the radial distance r, versus differing values of q and f. It is this probability-density representation that gives the more familiar 'dumbbell shaped' representations for the three aforesaid real 2p orbitals (Fig. 2.4).
To get a feel of the procedure for obtaining the boundary surface representations, let us attempt the two dimensional, on-paper plots corresponding to the actual (three-dimensional) boundary-surface representations for the 2pz orbital (as in Table 2.4) of the H-atom (Z = 1). Let the plane of your paper be the YOZ plane with y-axis be the vertical axis therein. So the coordinate q becomes the angle made by the line OP joining any on-paper point P and the origin O, with the rightward horizontal direction (Fig. 2.5). Let us now vary the angles q and f. For the 2pz orbital, f doesn't have any effect on y values (Table 2.4) and anywhere on the plane of our paper (i.e., the YOZ plane) f must equal ±90° = ±p/2 = ±1.571, so let us vary only q in steps of 10° (i.e., of p/18 radian) from its complete range 0° to 180°, fix the value of the variable r as 4ao (need to assume a sufficiently high fixed value of variable r, as mentioned in above paragraph, so that a major part of the electron probability gets included within the boundary surface regions obtained thereby), and get the values of |y(r,q,f)| and |y(r,q,f)|2 (as in Table 2.5 & Fig. 2.5 in another page) to be plotted along the radial distance. For the |y| plot, here is provided the (z, y) points to plot in a graph or in computer (if you use Microsoft Excel, remember to rearrange the horizontal-axis values in increasing order and to put the negative vertical-axial values in a third column); obtain them yourself for the |y|2 plot. Plotting them, you may verify that you indeed get the two 2pz plots shown in Fig 2.4!