There are many different electrolytes that CAN work in an electrolysis process, but here are the most common:
KOH- Potassium Hydroxide- Used in soap making
NaOH- Sodium Hydroxide- Lye- Used to open drains!
NaCI- Sodium chloride- Table Salt- Raises blood pressure
NaHCO- Baking Soda- Makes bubbles and poisonous gas
H2SO4- Sulfuric acid- Car battery acid- Makes bad gases and burns!
There are many other electrolytes in the acid, base and salt families, but in general these above are the main ones used in most Hydrogen electrolyzers.
Electrolysis involves the passage of an electric current
through, in general, an ionic substance that is either
molten or dissolved in a suitable solvent, resulting in chemical reactions at
the electrodes. The negative electrode is called
the cathode, and the positive electrode is the anode. [1] To be
useful for elctrolysis, the electrodes need to be able to conduct electricity,
and metal electrodes are generally used. Graphite electrodes and semiconductor
electrodes are also used. An ionic compound (or
covalently bonded in the case of acids) is dissolved with an appropriate solvent, or melted
by heat, so that its
ions are available in
the liquid. An electrical current is applied between a pair of inert electrodes immersed in the
liquid. Each electrode attracts ions that are of the opposite charge. Therefore,
positively-charged ions (called cations) move toward the
cathode, whereas negatively-charged ions (termed anions) move toward
the anode. The energy required to separate the ions, and cause them to gather at
the respective electrodes, is provided by an electrical power supply. At the
probes, electrons are absorbed or
released by the ions, forming a collection of the desired element or
compound.
Electrolytes commonly exist as solutions of acids, bases or salts. Furthermore, some
gases may act as
electrolytes under conditions of high temperature or low pressure. Electrolyte
solutions can also result from the dissolution of some biological (e.g. DNA, polypeptides) and synthetic
polymers (e.g. polystyrene
sulfonate), termed polyelectrolytes, which
contain multiple charged moieties.
Electrolyte solutions are normally formed when a salt is placed into a solvent such as water and the
individual components dissociate due to the thermodynamic interactions between
solvent and solute molecules, in a process
called solvation. For example, when
table salt, NaCl, is
placed in water, the following occurs:
- NaCl(s) → Na+ + Cl−
In simple terms, the electrolyte is a material that dissolves in water to
give a solution that conducts an electric current.
An electrolyte in a solution may be described as concentrated if it
has a high concentration of ions, or
dilute if it has a low concentration. If a high proportion of the
solute dissociates to form free
ions, the electrolyte is strong; if most of the solute does not
dissociate, the electrolyte is weak. The properties of electrolytes may
be exploited using electrolysis to extract
constituent elements and compounds contained
within the solution.
When electrodes are placed in an
electrolyte and a voltage is applied, the
electrolyte will conduct electricity. Lone electrons normally cannot pass
through the electrolyte; instead, a chemical reaction occurs at the cathode consuming
electrons from the cathode, and another reaction occurs at the anode producing electrons to be
taken up by the anode. As a result, a negative charge cloud develops in the
electrolyte around the cathode, and a positive charge develops around the anode.
The ions in the electrolyte move to neutralize these charges so that the
reactions can continue and the electrons can keep flowing.
For example, in a solution of ordinary salt (sodium chloride, NaCl)
in water, the cathode reaction will be
- 2H2O + 2e− → 2OH− + H2
and hydrogen gas will bubble up;
the anode reaction is
- 2H2O → O2 + 4H+ + 4e−
and oxygen gas
will be liberated. The positively charged sodium ions Na+ will move
toward the cathode neutralizing the negative charge of OH− there,
and the negatively charged chlorine ions Cl− will move towards the
anode neutralizing the positive charge of H+ there. Without the ions
from the electrolyte, the charges around the electrode would slow down continued
electron flow; diffusion of H+ and
OH− through water to the other electrode takes longer than movement
of the much more prevalent salt ions.
In other systems, the electrode reactions can involve the metals of the
electrodes as well as the ions of the electrolyte.
One important use of electrolysis of water is to produce hydrogen.
- 2H2O(l) → 2H2(g) + O2(g)
This has been suggested as a way of shifting society toward using hydrogen as
an energy carrier for
powering electric motors and internal combustion engines. (See hydrogen
economy.)
Electrolysis of water can be observed by passing direct current from a
battery or other DC power supply through a cup of water (in practice a saltwater
solution increases the reaction intensity making it easier to observe). Using platinum
electrodes, hydrogen gas will be seen to bubble up at the cathode, and oxygen will bubble
at the anode. If
other metals are used as the anode, there is a chance that the oxygen will react
with the anode instead of being released as a gas. For example, using iron
electrodes in a sodium chloride solution electrolyte, iron oxide will be
produced at the anode, which will react to form iron hydroxide. When producing
large quantities of hydrogen, this can significantly contaminate the
electrolytic cell - which is why iron is not used for commercial
electrolysis.
The energy efficiency of
water electrolysis varies widely. The efficiency is a measure of what fraction
of electrical energy used is actually contained within the hydrogen. Some of the
electrical energy is converted to heat, a useless by-product. Some reports quote
efficiencies between 50% and 70%[1]
This efficiency is based on the Lower Heating Value of Hydrogen. The Lower
Heating Value of Hydrogen is thermal energy released when hydrogen is combusted.
This does not represent the total amount of energy within the hydrogen, hence
the efficiency is lower than a more strict definition. Other reports quote the
theoretical maximum efficiency of electrolysis as being between 80% and 94%.[2]. The theoretical maximum considers the total amount of
energy absorbed by both the hydrogen and oxygen. These values refer only to the
efficiency of converting electrical energy into hydrogen's chemical energy. The
energy lost in generating the electricity is not included. For instance, when
considering a power plant that
converts the heat of nuclear reactions into hydrogen via electrolysis, the total
efficiency is more like 25%–40%.[3]
About four percent of hydrogen gas produced worldwide is created by
electrolysis, and normally used onsite. Hydrogen is used for the creation of
ammonia for fertilizer via the Haber process, and
converting heavy petroleum sources to lighter fractions via hydrocracking.
First law of electrolysis
In 1832, Michael Faraday reported
that the quantity of elements separated by passing an electrical current through
a molten or dissolved salt is proportional to the
quantity of electric charge passed through the circuit. This became the basis of
the first law of electrolysis:
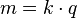
Second law of electrolysis
Faraday also discovered that the mass of the resulting separated
elements is directly proportional to the atomic masses of the
elements when an appropriate integral divisor is applied. This provided strong
evidence that discrete particles of matter exist as parts of the atoms of
elements.
Baking Soda (NaHCO#) is NOT an acceptable Electrolyte!
First, you would have to add 84 grams of baking
soda (NaHCO3) to
obtain the same amount of sodium as you would for 40 grams
of Sodium
Hydroxide (NaOH).
This is relevant because it is the Sodium
that is driving the
electrolysis process.
Secondly, on electrolysis of
NaHCO3, the Na+ ion will rush to the
cathode and you will get:-
2Na+ +
2e- + 2H2O -----> 2NaOH + H2
and
HCO3- + H2O -------> H2CO3
+ OH-
Also
H2CO3 --------> H2O + CO2
Also
CO2 +
2H+ + 2e- -----> CO + H2O
Also
CO + 2H+ + 2e- ------> C +
H2O
Conclusion: On adding NaHCO3 a whole range of chemical processes
can
take place but due to the nature of alkali metals, the one
sure
conclusion is that Hydroxides will be formed!
I believe Bob has
stressed this on a number of occasions and so people
should not be deceived
into thinking that if you make a completely
safe electrolytic solution using
NaHCO3 or other carbonates that you
end up with a completely safe
electrolytic solution after use!!
If one takes pH readings of the
electrolytic solution over time, one
can access the progress of the carbonate
solution (pH will increase
with increasing Alkalinity), but my advice is play
it safe, where PPE.
But when someone intentionally publishes that using
baking
soda is safe and does not put out carbon monoxide, then I have a
BIG
problem with that. I have done the tests, and I have had the
gas
analyzed. Sure, there is hydrogen, and sure, there is some CO2,
but
there is also enough CO to be lethal. There is NO oxygen
produced
until ALL of the carbon has been reacted from solution.
The
argument that the gas is to be burned and not inhaled does not fly with me!
How many of these people that use baking soda are
actually burning the gas
when they are doing their experiments? Most
are venting the gas into the air
in the room they are in, and even
those that DO burn the gas in an engine
often-times have leaks in
their systems.
I must confess that although I
posted a few of the reactions possible
in an electrolytic solution using this
substance, I had not realized
how favorable the reaction to CO was. If my
calculations are correct,
then a concentration of just 0.0667 % in the
atmosphere you are
breathing is enough to bind with 50 % of your Hemoglobin,
this is a
life threatening situation!!!
For non chemistry people who
wish to get a grasp of the toxicity of
Carbon Monoxide, a good rule of thumb
is, when you think Carbon
Monoxide, think Cyanide!
Some of the above information is from WIKIPEDIA and the other is from postings on the Yahoo "Hydroxy" website.
MORE TO COME STOP BACK- UNDER CONSTRUCTION!
Any questions or comments:
CONTACT
US!


