Quantum Mechanics
The Problem
At the end of the 19th Century, a problem had arisen with the theory of electromagnetism. All things that are warm give off heat as an electromagnetic wave. In formulating their theories scientists use an idealised object that radiates heat perfectly. This radiation is known as 'black box' radiation.
It was known that the wavelength of light is inversely proportional to its energy, i.e. the longer the wavelength the less energy the wave has. At any temperature, a range of radiation is given off, across the spectrum. According to classical physics, the radiation must come from the atoms vibrating, like vibrating a finger in a puddle. The faster the atom vibrates, the more energy that is contained in each wave. This would mean that shorter wavelengths would give off an increasing amount of energy with no upper limit. This would lead to infinite energy being given off. This is obviously wrong, and it didn't fit in with observation too For the majority of the spectrum, a simple relationship was shown, known as the Rayleigh-Jeans formula. The number of waves of short wavelengths is very small. A physicist, Wein, found a formula that could predict this, but it didn't have any 'reality' and it had to be added onto the Rayleigh-Jones law to describe the whole spectrum:
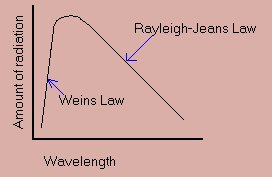
Max Planck became interested in this problem, and he managed to invent a formula that linked both together, but it was obviously concocted, so he carried on trying to find something that had more meaning. At that time, Boltzmann was working on entropy. He stated that entropy was statistical, so entropy could be reversed, but it was very improbable. Planck disagreed, but he tried using the same mathematics on the black-body problem. This involved splitting up the data into 'chunks' doing the calculations and then putting them back together (reintegrating them). Planck split the data into chunks, but noticed that gave the right answer, so he didn't reintegrate the data. This explained the graph excellently, and gave the formula:
E=h
nThis means Energy(E) of each wave is equal to the wavelength (n) multiplied by a constant (h). this constant is called Planck's constant (with a value of 6.6 x 10-34 Joule seconds). This meant that at small wavelengths the energy to produce one chunk was too great for many to be created, and as the wavelengths got very small, many were needed to make a difference to the amount of radiation.
Einstein joins in
It was a workable theory. It explained the black body radiation but, did it have any basis in reality? Einstein, in 1905, showed that it did. He examined the 'photoelectric' effect. This is where a light shone onto a surface will eject electrons. The strange thing was that the energy of the electrons ejected was not dependant on how strong the light was. It didn't matter if you used a small bulb or a huge lamp, the energy of the electrons was the same. The number of electrons would increase, but each of them would have the same energy. The way to increase the energy of the electrons was to change the colour of the light. This didn't make sense until Einstein used Planck's idea that light came in quanta, not in waves.
If a brighter light is used, more quanta are produced. So, more electrons are pushed of the material. But by using Planck's ideas, the energy of each quanta is dependant on the frequency of the waves. The colour determines the energy of the quanta and also the energy of the electrons being pushed off the material.
This was not accepted by all. It wasn't until 1916 that it was finally proven to be the case. This was what Einstein won his Nobel prize for.
Einstein though, did not like the implications of the theory, and spent the rest of his life trying to show that it was not a universal idea, but that there was another layer of reality behind it. He failed.
Is light a wave or a point?
The problem with this is that it had been proven experimentally that light is a wave. Now it was proven that light is a 'point'. These are contradictory. A wave is not a single point, it is 'smeared out' over its amplitude and wavelength. A single point cannot be a wave. This became known as the wave-particle duality. This strange hybrid is called a photon. It was noted also that not only do waves act like particles, but particles, such as electrons, act like waves.
One point, I think, illustrates this strange wave-particle duality and its importance to physics. In 1927 George Thompson won the Nobel prize for his experiment proving that electrons were waves. Earlier his father had won it for proving that electrons were particles! Both Nobel prizes are valid.
Now, electrons not being particles but waves had an important impact on our understanding of the atom. Neils Bohr discovered a new facet to our understanding of atomic structure can be explained by these ideas. From the study of chemistry we know that the first electron band has two electrons and all the rest have eight and that electrons can jump from level to level, either giving off a photon or receiving it. Now, if electrons behave like a snooker ball, under Newton's theories when they give off a photon they lose energy and so will slowly descend into the centre of the atom. This would make atoms unstable. If electrons are waves then the different valence levels correspond to whole number of electron wavelengths. So, there can be two electrons in the first valence level because there is one whole electron wavelength (two electrons in different states can be in the same valence level). Now, when an electron moves to a lower valence, it emits a quanta of energy corresponding to the energy lost. This quanta is of a specific energy value. An electron cannot exist between valence levels, because it cannot become half a wavelength.
The Double Slit experiment
What are the implications of all this? How do we know how true all of this is? Well, the best experiment to illustrate this is the double slit experiment.
A light source is set up that can either fire individual photons or be used as an ordinary light. In front of this is placed a board with two slits and in front of that a light sensitive board. Switch the light on and the beams of light go through both slits and, as waves, cause interference patterns on the board. Where the light waves coincide there are light patches. Where they are out of synchronisation by half a wavelength there is a dark patch. This is not surprising. Close one of the slits and a solid light patch appears on the board showing that there is no interference. Still no surprises.

Now, with just one slit open, send a single photon to the slit and repeat until a pattern appears on the board. There is obviously no interference. The next thing to do is open up both slits and then fire individual photons towards them. The pattern that emerges is one of the interference of waves. This is where the results started becoming strange.
This is an important point. Fire a single photon at the slits with one open and the pattern is just like it should be. Fire individual photons at the slits with both open and there is interference. Remember, these are individual photons. A photon goes through one slit and 'knows' whether the other slit is covered or not. If the slit that the photon does not go through is open, then somehow the photon seems to be aware of the fact and will only appear where it would if a continuos light is shone on it.
It is impossible to know where each individual photon will land but with the QM calculations the probabilities can be worked out. The photon can be thought of as a 'wave of potential'. Fire a photon and this probability wave fans out, and when the photon actually hits the screen the 'probability wave' collapses into the actual point. The measuring of the photon has become an intrinsic part of where the photon appears. It is impossible to know where it is, only where it could be, until it is observed. It has been shown that there is an intimate link between the observer and the thing observed. This became known as the Copenhagen Interpretation. It would seem that in all quantum processes, and remember, atomic particles are also quantum, nothing has an objective reality, it is all dependant on observation.
Schrodingers Cat
To understand this relationship between observation and reality, Erwin Schrodinger devised this thought experiment. Place a cat in a box and in the same box place a vial of poison. The vial of poison can be broken by a quantum process. There is a radioactive atom that on decaying will break the vial. Now, after a certain amount of time the atom has either decayed or not. This means that, because of the link between observation and event, the probability wave function (like the interference pattern from a single photon) hasn't collapsed and the cat is both dead and alive at the same time. As soon as the box is opened, the wave function collapses and the cat is either dead or alive.
The EPR Paradox
Einstein, as I have mentioned before, did not like the implications of Quantum Mechanics. He did not like the role of probability and chance. His most famous quote 'God does not play dice' came from his discussions about this. In 1935 Albert Einstein, Boris Podolsky and Nathan Rosen (the EPR comes from the initials of their surnames) invented a 'thought experiment' to show the limits of Quantum mechanics.
If a photon is divided into two smaller photons, each will be opposite to the other. One is left handed and the other right handed, to use the analogy. Now, if these two photons are separated without being observed, then they are in an indeterminate state. Each is both 'left' and 'right' at the same time. The soon one is observed, the state of the other one is determined instantly. Einstein and his colleagues said that was impossible. If the photons are separated by a long way, and one is looked at, the other instantly becomes the opposite, which means that there must have been some form of communication between them at faster than light speed, which according to relativity theory is impossible.
In the 1960's, John Bell suggested a way in which this could be tested, but it wasn't until the early 1980's when some French physicists under Alain Aspect showed that Quantum mechanics does violate relativity, there is a 'spooky action at a distance'. In 1997, Nikolas Gisin and his team in Geneva seperated two photons and sent them 10 Km apart before observing them, and the same weird thing was observed every time the experiment was repeated.
Heisenbergs uncertainty principle
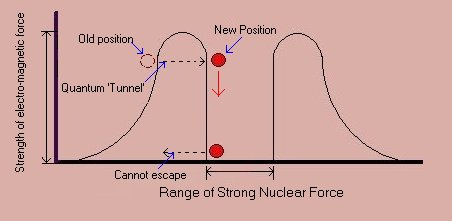
Werner Heisenberg pointed out another strange aspect of Quantum Mechanics. We cannot know exactly both the position and velocity of a particle. The more accurately we know its position the less we know of its velocity and vice versa. This is to the limit of Planck's constant. It is a fundamental restraint on the universe and it has been experimentally confirmed. The formation of Atom nuclei is made easier because of 'quantum tunnelling.' The strong nuclear force holds nuclei together, but it acts only across very short distances. A proton trying to get into a nucleus has to overcome the electrical repulsion of the other protons. Now, if it can get quite close to the nucleus, then, by the uncertainty principle, it is could possibly be the outside the nucleus (and being repelled by the electrical force) or inside (and being held in place by the strong nuclear force) so many can 'appear' inside the nucleus without the need to push as hard against the electrical force.
Virtual particles
Another major effect of the uncertainty principle is the fact that we cannot know both the energy of a particle and the amount of time it spends at that energy level. So, for short time spans, the energy level of a particle can become very great, in apparent contradiction of the first law of thermodynamics.
The consequence of this is that it is impossible to have a real vacuum. If there was a total vacuum the energy would be zero and the time at that state would also be known accurately. This is a violation of the uncertainty principle, so, at all times there is a flux of virtual particles. For very short time periods there appears a particle and its anti-particle which exist for a short time then cease to exist. The greater the energy of the virtual particles the shorter the time they exist.
Further reading:
'The Arrow of Time' by Peter Coveney and Roger Highfield
'A Brief History of Time' by Stephen Hawking
'In search of Schrodingers Cat' by John Gribbon