TIP ETCHING
ETCHING TUNGSTEN TIPS
Electro-chemically etched tips display characteristics of greater sharpness and of uniformity. They do this more reliably than do tips that have been cut by shears. And tungsten, one of the more commonly used tip materials, is so hard that it would damage ordinary shears.
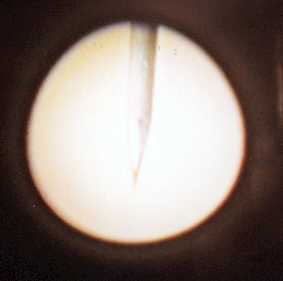
The image captured above shows the setup for etching tungsten by the lamellar method. This technique restricts the electrolyte to a thin membrane around the area to be etched. The outer -ve electrode is a ring, 10mm in diameter, made from steel wire. This is dipped, with the ring horizontal, into 2-Molar acqueous sodium hydroxide (NaOH) and gently lifting a membrane of solution. A length of tungsten wire, with a diameter of 0.25mm, is pushed through the centre of the membrane and held in place there. This will be the +ve electrode.
A potential of about 3volts to 5volts is applied to the electrodes. The etching current starts off relatively high, about 10mA. But as the tungsten necks off and the electrolyte looses some of its conductivity, the current falls to around 1mA. Finally, after about 20 minutes, the lower part of the etched wire drops off into the tip catcher. The etched tip-wire is 7mm long. The tip catcher sides are shorter than this, so there is no contact with the tip itself. The extreme end of the tip is obviously very delicate and totally untouchable.
The one big disadvantage with etching techniques similar to this is that the tip is covered with a thin layer of oxide. If this layer is not removed, tunneling conditions will be unstable and a proper scan simply not possible. The oxide layer can sometimes be removed by performing a controlled tip crash into the sample surface. However such action can greatly affect tip sharpness, and for certain types of sample, any particles picked up from the surface will have undesirable effects. The standard technique for oxide removal for tungsten tips is by heating to 600C in vacuum. As I do not have access to the kind of apparatus that can be used for annealing, I am presently using chemical means.
WARNING! HYDROFLUORIC ACID IS HIGHLY CORROSIVE . . . IT SHOULD NOT COME IN CONTACT WITH SKIN. A good way to remove the oxide is to dip the tip in concentrated hydrofluoric acid for 10 to 30 seconds. The tip can then be rinsed with methanol to remove traces of the acid. The tip is held downwards with tweezers and the alcohol allowed to run down the wire and off the end. Obviously, only a very small quantity of the acid is required. However one must always be aware that there are serious safety issues regarding the use of HF. And one must also remember that it attacks glass, and must therfore be stored in a container made of plastic or stainless steel.
FIELD EVAPORATION
Field emission is the process by which a flow of electrons can be emitted from a sharp object by building a high voltage potential across a tiny gap. Some atoms get dislodged by the emission current and this process can be used to re-shape a slightly blunted tip. The STM itself can be used to position the tip about 10 micrometers away from a conductive surface. The electrometer amplifier is completely disconnected to avoid it being damaged. A 300V D.C supply is applied between the tip and conductor, (the tip being at the -ve potential), and the current monitored.
Certain literature advises that for effective field evaporation, the current across the gap should be in the order of a few microamperes. The Fowler-Nordheim equation dictates that this current is both a function of electrical field strength and the effective radius of the tip. Therefore the treshold value of the field emission current can serve as a gauge of tip sharpness.
HV power supply