
 Causes
CausesThe ozone layer is easily damaged by chemicals used in the industry. The chemicals break down the ozone, and this allows the harmful rays of the sun to reach the earth. There are 3 different types of UV (ultraviolet) light that come from the sun, and luckily, the ozone layer in the earth's atmosphere absorbs a lot of it, as shown in the picture below.
↓Picture obtained from http://coolshade.tamu.edu/uv_4.html
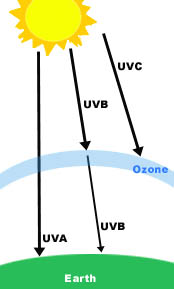
These 3 types are:
If the UVA and UVB reaching the earth's surface, and your skin, you could develop a deadly skin cancer.
When volcanoes erupt,
they release hundreds of tons of water vapor and chemicals into the atmosphere.
The chemicals include hydrogen chloride, which is a mixture of hydrogen and
chlorine atoms. The more powerful eruptions send a cloud of water vapor and
chemicals beyond the troposphere and into the stratosphere. This causes the
ozone layer to decay in thickness. Also, scientists have discovered that ice in
the troposphere removed most hydrogen chloride from the volcanic clouds, but a
small amount reached the ozone layer. The resulting thinning of the ozone layer
lasted a few years. 
CFC's (chlorofluorocarbons) cause a damaging effect on the ozone layer.
Chlorofluorocarbons (CFCs) contain atoms of chlorine, fluorine, and carbon that are arranged in a stable chemical structure. They were invented in the 1920s and have been used for propellants, refrigerants, and foaming agents. Manufactures have unintentionally used CFCs for decades because of their stability and that they are not reactive with other atmospheric elements in the lower atmosphere. The manufactures did not know at the time, but the CFCs were actually finding their way into higher parts of the atmosphere. It wasn’t until the 1970s that humans were finally aware of their destructive nature in the stratosphere.
The CFC molecules are released from aerosol cans, toxic fumes, waste plants and things like that. CFC molecules don't break apart until they reach the ozone layer (which is about 10 yrs after they are released) and when they do, chlorine and bromine atoms escape. In certain conditions, the chlorine and bromine atoms attack the ozone molecules, so then ozone is being depleted by the hundreds. Scientists discovered in 1974 that these molecules float up into the atmosphere. Recent findings show that there has been a 2-3% loss of ozone in the stratosphere, which is much more than what was thought. Since 1979, the growth of the ozone hole is now as wide as the United States and as deep as Mount Everest.
Other things that effect the ozone layer are lightning flashes through the sky, both creates ozone from oxygen and makes some chemicals in the air to attack the ozone. Also, when there is a forest fire, they release gases into the air. These gases can drift upwards and damage the ozone layer.
Because CFC's remain in the atmosphere for centuries, it will take a very long time for the ozone layer to repair itself and return back to the way it was.
Over millions of years, living things have evolved to resist very specific amounts of UV radiation. Each time the ozone decreases by 1%, the amount of UV that reaches the earths surface increases by 2%. For each 1% increase in UV, there is an expected 2% increase of skin cancer rates.
Because the ozone hole is getting bigger, the amount of UV
rays that enter the earth's atmosphere is growing. UV (ultraviolet) rays is
electromagnetic radiation at wavelengths shorter than the violet end of visible
light; the atmosphere of the earth effectively blocks the conduction of most
of the ultraviolet light. Some things that could happen
to us because of this massive amount of UV light entering our atmosphere are: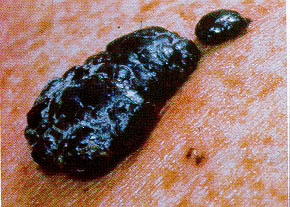
So in short, the increased rates of ozone depletion are damaging the entire food chain,
_____________________________________________________________________