
Among the most important of the biological molecules are the carbohydrates. Like the previous biomolecules studied in this course, carbohydrates are a large reason for life as we know it. Carbohydrates provide a source of energy for organisms and also play structural role in some creatures. These molecules, also known as saccharides, are constituted of the empirical formula (CH2O)n. Like previous biomolecules studied, such as the proteins and nucleic acids, individual units are important, as are the polymers of these units. In this page I will summarize carbohydrates, giving a framework of understanding for the remainder of this project. I will begin by defining some commonly used terms. (Definitions from Biochemistry, Mathews and van Holde.)
Aldose: a monosaccharide aldehyde.
Diastereoisomers: molecules that are stereoisomers but not enantiomers. Isomers that differ in configuration about two or more asymmetric carbon atoms and are not complete mirror images. Have one or more chiral carbons. (Remember: a molecule with n chiral centers has 2n stereoisomers.)
Enantiomers: stereoisomers that are nonsuperimposable mirror images of each other. Also known as optical isomers, based on the fact that the enantiomers of a compound rotate polarized light in opposite directions.
Ketose: a monosaccharide ketone.
Metabolism:The totality of the chemical reactions that occur in an organism.
Monosaccharides: the simplest carbohydrates; small; monomeric. Includes glucose.
Oligosaccharides: A carbohydrate composed of a few monomer units. Includes maltose, a disaccharide (two glucose units).
Photosynthesis: The process by which energy from light is captured and used to drive the synthesis of carbohydrates from CO2 and H2O. The process of photosynthesis is essential for all living things in the world, and plants are the only food-producers, while the other animals either feed on plants or feed on other animals. It is an oxidation-reduction reaction. The overall reaction: (CO2(g))x + (H2O(l))y + light energy ------> Cx(H2O)y (aq) + O2 x (g)
Polysaccharides: Polymers of monosaccharides, can be quite complex. Includes amylose (starch).
Tautomer: Structural isomers that have different location of their hydrogen atoms and double bonds.
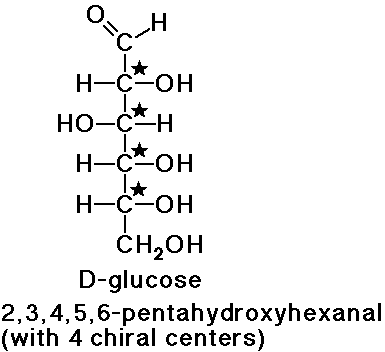 A Glucose Molecule
A Glucose MoleculeThe smallest monosaccharides we will discuss here have n=3, the trioses. There are two trioses, which are also tautomers - glyceraldehyde (an aldose) and dihydroxyacetone (a ketose). These two molecules are capable of interconversion through an enediol intermediate. Glyceraldehyde exists in enantiomer (D & L) forms, because it has a chiral carbon (second carbon). The D form predominates in nature.
Tetroses have n = 4, and have two chiral carbons in the aldose form. Because there are 2n stereoisomers for every chiral carbon, there are four stereoisomers for every aldotetrose.
For these molecules (with n=5 and n=6, respectively) the most common form under physiological conditions is the ring structure. Pentoses (non-ring) have three chiral carbons, and thus eight stereoisomers. Hexoses have a large number of possible conformations,. Almost all of the hexoses are important biologically, especially glucose (blood sugar) and fructose (fruit sugar). Glucose is the bulding block of polysaccharides.
Of all of the oligosaccharides, disaccharides are the most biologically important. They are composed of two monosaccharide units. Example molecules are sucrose, lactose and maltose.
These molecules are the U-hauls of life - they perform storage functions, such as in the case of starch. Polysaccharides are also the bricks of many organisms - they serve as structural units, such as the chitin and cellulose of plants, or peptidoglycan of bacterial cell walls. Another example is glycogen, a storage polysaccharide of animals and microbes.
As in proteins, the primary sequence of the monomer units determines the structure of the polysaccharide. If a polysaccharide consists of all of the same monomer units, it is a homopolysaccharide. When the monomer units vary, it is a heteropolysaccharide.
To go to an excellent page on Nomenclature and Stereochemistry of carbohydrates, visit this page from Iowa State.
This page, from the United Kingdom, features a Monosaccharide Browser with space filling Fischer Projections.
Need to review your functional groups? Or would you like to see an overview of carbohydrates? Visit the Buglady.
To study the chemistry of biopolymers, including all of the major ones studied in this class, go to visit this great review page.
An excellent reference page, with links to many major journals, is put out by Harvard University
Carbohydrates are an essential part of our diet. However, there is always the worry of too much of a good thing. New diet drugs are constantly under research and testing that are intended to decrease our desire for the sweet stuff. Further, recent research has disproven that hyperactivity in children in linked to monosaccharide consumption.
Project-Related Links
Back to Home Page
Metabolism
Carbohydrate Functions
Photosynthesis
Food for Thought...